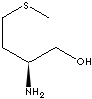
| L-METHIONINOL | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 2899-37-8 |
|
| EINECS NO. | 220-788-1 | |
| FORMULA | C5H13NOS | |
| MOL WT. | 135.22 | |
|
H.S. CODE |
||
| TOXICITY |
|
|
| SYNONYMS | L-2-Amino-4-methylthiobutan-1-ol; | |
| L-2-Amino-4-metiltiobutan-1-ol; L-2-Amino-4-méthylthiobutane-1-ol; (S)-(-)-2-amino-4-methylthio-1-butanol; | ||
|
DERIVATION |
|
|
|
CLASSIFICATION |
||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE |
clear crystals |
|
| MELTING POINT | 31 - 33 C | |
| BOILING POINT | 198 - 200 C | |
| SPECIFIC GRAVITY | 1.09 - 1.10 | |
| SOLUBILITY IN WATER | ||
| pH | ||
| VAPOR DENSITY |
|
|
|
AUTOIGNITION |
||
|
NFPA RATINGS |
||
|
REFRACTIVE INDEX |
1.4511 | |
| FLASH POINT |
90 C |
|
| STABILITY |
Stable under ordinary conditions. Hygroscopic. |
|
|
GENERAL DESCRIPTION |
||
Chiral amino
alcohols and their N-protected derivatives (Cbz, t-Boc, and Fmoc)
are versatile building blocks, auxiliaries, ligand
or resolving agent in asymmetric synthesis for biological research and
pharmaceutical applications such as antitumor, anesthetic, antipasmodic,
hepatotoxic, antiinflammatory or anti-HIV activities. They are used in the
synthesis of beta-blockers, HIV protease and ACE inhibitors, chiral auxiliaries
and catalytic enantioselective ligands. Their derivatives include the forms
of;
|
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
clear crystals |
|
|
CHEMICAL PURITY |
97.5% min | |
|
OPTICAL PURITY |
97.5% min | |
| OPTICAL ROTATION | -20° ~ -22° (C=5 in Water)) | |
| TRANSPORTATION | ||
| PACKING |
|
|
| HAZARD CLASS | ||
| UN NO. | ||
| OTHER INFORMATION | ||
| Hazard Symbols: XI, Risk Phrases: 36/37/38, Safety Phrases: 22-24/26 | ||