|
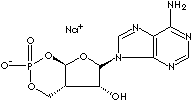
ADENOSINE-3',5'-CYCLIC MONOPHOSPHATE, SODIUM SALT | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. |
37839-81-9 |
|
| EINECS NO. | ||
| FORMULA | C10H12N5O6P·Na | |
| MOL WT. | 477.39 | |
|
H.S. CODE |
|
|
|
TOXICITY |
||
| SYNONYMS |
cAMP, Sodium; Cyclic adenylic acid, Sodium; |
|
|
3',5'-AMP Sodium Salt; Cyclic 3',5'-(Hydrogen Phosphate)Adenosine Monosodium Salt; Cyclic Adenylic acid, Sodium; |
||
| DERIVATION |
|
|
|
CLASSIFICATION |
||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE |
white powder | |
| MELTING POINT | 219 - 220 C | |
| BOILING POINT |
| |
| SPECIFIC GRAVITY | ||
| SOLUBILITY IN WATER |
Soluble | |
| pH | ||
| VAPOR DENSITY |
| |
|
AUTOIGNITION |
||
|
NFPA RATINGS |
||
|
REFRACTIVE INDEX |
| |
| FLASH POINT |
| |
| STABILITY |
Stable under ordinary conditions | |
|
GENERAL DESCRIPTION & APPLICATIONS |
||
|
Adenosine
: a purine nucleoside composed of adenine linked by its N9
nitrogen to the C1 carbon of ribose. It is a component of ribonucleic acid and
its nucleotides (AMP., ADP, ATP, cAMP) play important roles in biochemical
processes such as synthesis of nucleic acids and proteins, photosynthesis,
muscle contraction and intracellular signal transduction (cAMP). AMP., ADP, ATP
are three interconvertible compounds in which adenosine is attached through its
ribose group to one (monophosphate), two (diphosphate), and three (triphosphate)
phosphoric acid molecules.
Adenosine Triphosphate (ATP) : a nucleotide composed of adenine, the sugar ribose, and three phosphate groups; involved in energy metabolism and required for RNA synthesis. It exists in cells in a form of high-energy phosphate bond to store and transport chemical energy. The pyrophosphate nature of the bonds between ATP's three phosphate radicals results in a powerful donor of phosphate groups to suitable acceptors. When it is broken down by hydrolysis, it yields ADP (adenosine diphosphate), inorganic phosphorus, and energy. The free energy derived from hydrolysis of ATP is used to drive metabolic reactions including the synthesis of nucleic acids and proteins, to move molecules against concentration gradients (active transport), and to produce mechanical motion (contraction of microfibrils and microtubules). ADP can be further broken down to yield adenosine monophosphate (AMP), additional phosphorus, and more energy. When the phosphorus and energy are immediately used to drive other reactions, such as the synthesis of UDP (uridine diphosphate), an RNA precursor, from UMP (uridine monophosphate), the pair of reactions are said to be coupled. New ATP is produced from AMP using the energy released from the breakdown of fuel molecules, such as fat and glucose which is broken down into pyruvate in the cytosol. Two molecules of ATP are generated for each molecule of glucose. ADT can be converted back to ATP by the processes of oxidative phosphorylation and substrate-level phosphorylation. Adenosine Diphosphate (ADP) : a nucleotide composed of pyrophosphate of adenosine, involved in energy metabolism; it is produced by hydrolysis of ATP and converted back to ATP by the processes of oxidative phosphorylation and substrate-level phosphorylation. Adenosine Monophosphate (AMP, also called adenylic acid.) : a nucleotide, 5'-phosphate of adenosine, produced by the hydrolysis of ATP and converted to ADP by adenylate kinase. Involved in the reactions of intracellular energy transfers. Cyclic Adenosine Monophosphate (cAMP)
: cyclic AMP containing an
additional ester linkage between the phosphate and ribose units; serves as an
intracellular and, in some cases, extracellular secondary messenger mediating
the action of many peptide or amine hormones. It also plays a role in the
transcription of some genes.
Deoxyadenosine (dA) : a purine nucleoside composed of adenine linked by its N9 nitrogen to the C1 carbon of deoxyribose. (deoxy-, also called desoxy, is a prefix for the designation of compounds which contain one less atom of oxygen than the reference substance). Deoxyadenosine diphosphate (dADP) : a nucleotide, 5'-pyrophosphate of deoxyadenosine. Deoxyadenosine monophosphate (dAMP) : a nucleotide, 5¢-phosphate of deoxyadenosine, occurring in deoxyribonucleic acid. Deoxyadenosine triphosphate (dATP): a nucleotide, the 5'-triphosphate of deoxyadenosine; activated precursor in DNA synthesis. |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
white powder |
|
|
PURITY (HPLC) |
98.0% min |
|
| TRANSPORTATION | ||
| PACKING | 100mg,
1g | |
| HAZARD CLASS | Not regulated | |
| UN NO. | ||
| OTHER INFORMATION | ||
| Ribose is a pentose (five-carbon sugar) that is a component of the ribonucleic acid (RNA), where it alternates with phosphate groups to form the 'back-bone' of the RNA polymer and binds to nitrogenous bases. Ribose phosphates are components of the nucleotide coenzymes and are utilized by microorganisms in the synthesis of the amino acid histidine. Its close relative, deoxyribose, is a constituent of deoxyribonucleic acid (DNA), where it alternates with phosphate groups to form the 'back-bone' of the DNA polymer and binds to nitrogenous bases. The presence of deoxyribose instead of ribose is one difference between DNA and RNA. Ribose has one more oxygen atom in its molecule than deoxyribose. Ribose has a five member ring composed of four carbon atoms and one oxygen. Hydroxyl groups are attached to three of the carbons. The other carbon and a hydroxyl group are attached to one of the carbon atoms adjacent to the oxygen. In deoxyribose, the carbon furthest from the attached carbon is stripped of the oxygen atom in what would be a hydroxyl group in ribose. The sugar (ribose or deoxyribose) molecules in the nucleic acid are all oriented in the same direction. Their carbon atoms are numbered: the 5' carbon atom is always on the side of the sugar molecule that faces the leading end, while the 3' carbon atom always faces the tail end. Nucleotide is the structural unit of a nucleic acid. A nucleotide consists of either a nitrogenous heterocyclic base (purine or pyrimidine) , a pentose sugar (ribose or deoxyribose) and a phosphate group attached at the 5' position on the sugar. A nucleoside consists of only a pentose sugar linked to a purine or pyrimidine base, without a phosphate group. Purine bases are Adenine, Guanine and Hypoxanthine (examples of purine nucleosides are Adenosine, 2'-Deoxyadenosine, Guanosine, 2'-Deoxyguanosine, Inosine, 2'-Deoxyinosine). Pyrimidine bases are Cytosine, Thymine, and Uracil (examples of pyrimidine nucleosides are Cytidine, 2'-Deoxyguanosine, 5-Methyluridine, 2'-Deoxy-5-Methyluridine, Uridine, 2'-Deoxyuridine). The nucleoside derivatives are involved in important functions in cellular metabolism and are used to synthesize enzyme inhibitors, antiviral agents, and anticancer agents. | ||