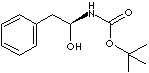
| N-tert-BOC-L-PHENYLALANINOL | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 66605-57-0 |
|
| EINECS NO. | ||
| FORMULA | C6H5CH2CH[NHCO2C(CH3)3]CH2OH | |
| MOL WT. | 251.32 | |
|
H.S. CODE |
||
| TOXICITY |
|
|
| SYNONYMS | N-t-Butoxycarbonyl-L-phenylalaninol; N-Boc-L-Phenylalaninol | |
| Boc-(S)-2-amino-3-phenyl-1-propanol; N-t-Butoxycarbonyl-L-phenylalaninol; (S)-2-(BOC-amino)-3-phenyl-1-propanol; N-(tert-Butoxycarbonyl)-L-phenylalaninol; | ||
|
DERIVATION |
|
|
|
CLASSIFICATION |
||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE |
white to off-white powder |
|
| MELTING POINT | 94 - 96 C | |
| BOILING POINT | ||
| SPECIFIC GRAVITY | ||
| SOLUBILITY IN WATER | Insoluble | |
| pH | ||
| VAPOR DENSITY |
|
|
|
AUTOIGNITION |
||
|
NFPA RATINGS |
||
|
REFRACTIVE INDEX |
||
| FLASH POINT |
|
|
| STABILITY |
Stable under ordinary conditions. |
|
|
GENERAL DESCRIPTION |
||
|
Chiral amino alcohol possessing both amine and alcohol is a structural motif for the compounds which have important biological and pharmacological functions within the body as inhibitor of aspartyl proteases, aldose reductase, b-amyloid peptide formation, and dopamine D4 antagonists. The chiral amino alcohol also exhibits anti-inflammatory, anesthetic, antipasmodic, hepatotoxic, anti-diabetic, anti-obesity,and anti-depressant activities. Chiral amino alcohols and their N-protected derivatives (Cbz, t-Boc, and Fmoc) are versatile building blocks, auxiliaries, ligand or resolving agent in asymmetric synthesis for biological and pharmaceutical researches. applications are present in the field of antitumor, anesthetic, antipasmodic, hepatotoxic, antiinflammatory or anti-HIV activities. Their derivatives include the forms of;
|
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
white to off-white powder |
|
|
CHEMICAL PURITY |
98.0% min | |
|
OPTICAL PURITY |
98.0% min | |
| OPTICAL ROTATION | -25° ~ -29° (C=1, CHCl3) | |
| TRANSPORTATION | ||
| PACKING |
|
|
| HAZARD CLASS | ||
| UN NO. | ||
| OTHER INFORMATION | ||
| Hazard Symbols: XI, Risk Phrases: 36/37/38, Safety Phrases: 22-24/26 | ||