|
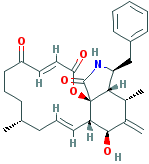
CYTOCHALASIN A |
| Synonyms. Cytochalasin A; 7(S)-Hydroxy-16(R)-methyl-10-phenyl-24-oxa(14)cytochalasa-6(12),13(E),21(E)-triene-1,20,23-trione; 5,5-Didehydrophomin; 5-Dehydrophomin; Dehydrophomin; (7S,13E,16R,21E)-7-Hydroxy-16-methyl-10-phenyl-24-oxa[14]cytochalasa-6(12),13,21-triene-1,20,23- trione; 16-Benzyl-13-hydroxy-9,15-dimethyl-14-methylene-8,9,10,12a,13,14,15,15a,16,17-decahydro-2H- oxacyclotetradecino [2,3-d]isoindole -2,6,18(5H,7H)-trione; |
|
|
| PRODUCT IDENTIFICATION | |
|
CAS RN |
14110-64-6 |
|
EINECS RN |
237-964-9 |
|
FORMULA |
C29H35NO5 |
|
MOLE WEIGHT |
477.59 |
|
H.S CODE |
2934.99.3900 |
|
SMILES |
c1cc(ccc1)C[C@@H]1NC(=O)[C@]23[C@H]1[C@@H](C([C@H]([C@@H] 2C = CC[C@@H](CCCC(=O)C=CC(=O)O3)C)O)=C)C |
|
CLASSIFICATION |
Mycotoxin, Actin inhibitor |
|
EXTRA NOTES |
Other RN: 11032-94-3, 11041-99-9 |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES | |
|
PHYSICAL STATE. |
white powder |
|
MELTING POINT |
193 ~ 195 C |
|
BOILING POINT |
|
|
DENSITY |
|
|
SOLUBILITY IN WATER |
|
| SOLVENT SOLUBILITY |
|
|
VAPOR DENSITY |
|
|
log P(octanol-water) |
|
|
VAPOR PRESSURE |
|
|
AUTOIGNITION TEMP |
|
| pK |
|
|
REFRACTIVE INDEX |
|
|
FLASH POINT |
|
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions. |
|
INCOMPATIBLE MATERIALS |
Strong oxidizing agents, Strong bases, Strong acids |
| POLYMERIZATION |
Has not been reported |
|
NFPA RATINGS |
Health: 4, Flammability: 0, Reactivity: 0 |
|
|
| EXTERNAL LINKS & GENERAL DESCRIPTION |
|
USA.gov - Cytochalasin A Wikipedia Linking - Cytochalasin Google Scholar Search - Cytochalasin A U.S. National Library of Medicine - Cytochalasin A PubChem Compound Summary - Cytochalasin A KEGG (Kyoto Encyclopedia of Genes and Genomes) - Cytochalasin A NCBI (http://www.ncbi.nlm.nih.gov/) - Cytochalasin A EPA - Substance Registry Services - Cytochalasin A The Cytochalasins (Greek cytos, cell; chalasis, relaxation) are a group of related fungal metabolites. They were discovered in 1964 during the screening of mold filtrates for possible biological activity on cells. These fungal toxins are related by chemical structure. All are characterized by a highly substituted hydrogenated isoindole ring to which is fused a macrocyclic ring. The macrocyclic ring may vary from 11–14 atoms and may be either a carbocycle or lactone. These fungal toxins also share a number of unusual, interesting, and characteristic effects on the animal cell. sigmaaldrich |
|
|
| SALES SPECIFICATION | |
|
APPEARANCE |
white powder |
|
ASSAY |
98% min |
|
MELTING POINT |
193 ~ 195 C |
|
LOSS ON DRYING |
1% max |
|
|
| TRANSPORT & REGULATORY INFORMATION | |
|
UN NO. |
1544 |
| HAZARD CLASS |
6.1 |
| PACKING GROUP | II |
|
|
| SAFETY INFORMATION | |
|
HAZARD OVERVIEW |
GHS (Globally Harmonised System) Classification: Acute toxicity (Oral). Acute toxicity (Inhalation). Acute toxicity (Dermal). Reproductive toxicity. Hazard statements: Fatal if swallowed or in contact with skin. Fatal if inhaled. Suspected of damaging fertility or the unborn child. |
| SIGNAL WORD | Danger |
|
PICTOGRAMS |
|
|
HAZARD STATEMENTS |
H300 + H310-H330-H361 |
|
P STATEMENTS |
P260-P264-P280-P284-P302 + P350-P310 |
| EC DIRECTIVES |
|
| HAZARD CODES |
|
|
RISK PHRASES |
63-26/27/28 |
|
SAFETY PHRASES |
28-36/37-45 |
|
|
| PACKING |
|
Preserve in light-resistant and well-closed containers |
|
|