|
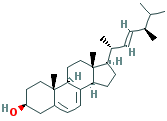
ERGOSTEROL |
| Synonyms. Ergosterol; 24-Methylcholesta-5,7,22-trien-3beta-ol; 24alpha-Methyl-22E-dehydrocholesterol; 24R-Methylcholesta-5,7,2E-trien-3beta-ol; Ergosta-5,7,22-trien-3beta-ol; Ergosterin; Provitamin D; |
|
|
| PRODUCT IDENTIFICATION | |
|
CAS RN |
57-87-4 |
|
EINECS RN |
200-352-7 |
|
FORMULA |
C28H44O |
|
MOLE WEIGHT |
396.65 |
|
H.S CODE |
2936.29.5020 |
|
SMILES |
C[C@H](/C=C/[C@H](C)C(C)C)[C@H]1CC[C@@H]2[C@@]1(CC[C@H] 3C 2=CC=C4[C@@]3 (CC[C@@H] (C4)O)C)C |
|
CLASSIFICATION |
Sterol, Vitamin D |
|
EXTRA NOTES |
A steroid of interest both because its biosynthesis in FUNGI is a target of ANTIFUNGAL AGENTS, notably AZOLES, and because when it is present in SKIN of animals, ULTRAVIOLET RAYS break a bond to result in ERGOCALCIFEROL. MeSH |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES | |
|
PHYSICAL STATE |
white to off-white crystalline powder |
|
MELTING POINT |
156 ~ 158 C |
|
BOILING POINT |
250 c |
|
DENSITY |
|
|
SOLUBILITY IN WATER |
Insoluble |
| SOLVENT SOLUBILITY |
|
|
VAPOR DENSITY |
|
|
log P(octanol-water) |
|
|
VAPOR PRESSURE |
|
|
AUTOIGNITION TEMP |
|
| pK |
|
|
REFRACTIVE INDEX |
|
|
FLASH POINT |
|
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions. |
|
INCOMPATIBLE MATERIALS |
Strong oxidizing agents. Acids |
| POLYMERIZATION |
Has not been reported |
|
NFPA RATINGS |
Health: 0, Flammability: 0, Reactivity: 0 |
|
|
| EXTERNAL LINKS & GENERAL DESCRIPTION |
|
USA.gov - Ergosterol Wikipedia Linking - Ergosterol Google Scholar Search - Ergosterol U.S. National Library of Medicine - Ergosterol PubChem Compound Summary - Ergosterol KEGG (Kyoto Encyclopedia of Genes and Genomes) - Ergosterol ChEBI (http://www.ebi.ac.uk/chebi/) - Ergosterol NCBI (http://www.ncbi.nlm.nih.gov/) - Ergosterol Material Safety Data Sheet - Ergosterol Human Metabolome Database - Ergosterol Hazardous Substances Data Bank - Ergosterol Ergosterol is a biological precursor of Vitamin D2 found in cell membranes of fungi and some protists such as trypanosomes. Ergosterol may be used to study the function of anti-fungal drugs such as Amphotericin B and its analogues and to study the ergosterol biosynthesis pathway within various fungi. sigmaaldrich |
|
|
| SALES SPECIFICATION | |
|
APPEARANCE |
white to off-white crystalline powder |
|
ASSAY |
98% min |
|
MELTING POINT |
156 ~ 158 C |
| OPTICAL ROTATION |
-119° ~ -121° (c=1, chloroform) |
|
LOSS ON DRYING |
1% max |
|
HEAVY METALS |
10ppm max |
|
|
| TRANSPORT & REGULATORY INFORMATION | |
|
UN NO. |
2811 |
| HAZARD CLASS |
6.1 |
| PACKING GROUP | II |
|
|
| SAFETY INFORMATION | |
|
HAZARD OVERVIEW |
GHS (Globally Harmonised System) Classification: Chronic aquatic toxicity. Hazard statements: May cause long lasting harmful effects to aquatic life. |
| SIGNAL WORD | Warning |
|
PICTOGRAMS |
|
|
HAZARD STATEMENTS |
H13 |
|
P STATEMENTS |
|
|
|
| PACKING |
|
Preserved in light-resistant and well-closed bottles |
|
|