|
MIMOSINE |
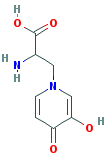
| Synonyms. Mimosine; (L)-Mimosine; (S)-alpha-Amino-3-hydroxy-4-oxo-1(4H)-pyridylpropionic acid; Leucenol; (2S)-2-Amino-3-(3-hydroxy-4-oxopyridin-1-yl)propanoic acid; beta-[N-(3-Hydroxy-4-pyridone)]-alpha-aminopropionic acid; |
|
|
| PRODUCT IDENTIFICATION | |
|
CAS RN |
500-44-7 |
|
EINECS RN |
207-905-1 |
|
FORMULA |
C8H10N2O4 |
|
MOLE WEIGHT |
198.18 |
|
H.S CODE |
2933.39.6190 |
|
SMILES |
c1(c(=O)ccn(c1)C[C@H](N)C(=O)O)O |
|
CLASSIFICATION |
Alkaloid |
|
EXTRA NOTES |
3-Hydroxy-4-oxo-1(4H)-pyridinealanine. An antineoplastic alanine-substituted pyridine derivative isolated from Leucena glauca MeSH |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES | |
|
PHYSICAL STATE. |
white to off-white crystalline powder |
|
MELTING POINT |
228 C |
|
BOILING POINT |
|
|
DENSITY |
|
|
SOLUBILITY IN WATER |
|
| SOLVENT SOLUBILITY |
|
|
VAPOR DENSITY |
|
|
log P(octanol-water) |
-4.72 |
|
VAPOR PRESSURE |
|
|
AUTOIGNITION TEMP |
|
| pK |
|
|
REFRACTIVE INDEX |
|
|
FLASH POINT |
|
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions. |
|
INCOMPATIBLE MATERIALS |
Strong oxidizing agents |
| POLYMERIZATION |
Has not been reported |
|
NFPA RATINGS |
Health: 1, Flammability: 0, Reactivity: 0 |
|
|
| EXTERNAL LINKS & GENERAL DESCRIPTION |
|
USA.gov - Mimosine Wikipedia Linking - Mimosine Google Scholar Search - Mimosine U.S. National Library of Medicine - Mimosine PubChem Compound Summary - Mimosine Drug Bank - Mimosine KEGG (Kyoto Encyclopedia of Genes and Genomes) - Mimosine ChEBI (http://www.ebi.ac.uk/chebi/) - Mimosine NCBI (http://www.ncbi.nlm.nih.gov/) - Mimosine Hazardous Substances Data Bank - Mimosine EPA - Substance Registry Services - Mimosine Biochem/physiol Actions L-Mimosine is a plant amino acid and potential inhibitor of the cell cycle giving rise to growth arrest in G1-phase. An iron chelator that inhibits DNA replication in mammalian cells. Has been shown to have apoptotic activity in xenotransplanted human pancreatic cancer. L-Mimosine is also used as a hypoxia-mimetic agent to stabilize Hypoxia Inducible Factor 1 (HIF-1). L-Mimosine stabilizes HIF-1 through the inhibition of Prolyl Hydroxylases (PHDs) which target HIF-1 through degradation. The mechanism of inhibition is likely through the chelation of Fe2+ bound to the active site of PHD which is required for enzymatic activity. sigmaaldrich |
|
|
| SALES SPECIFICATION (L-Form) | |
|
APPEARANCE |
white to off-white crystalline powder |
|
ASSAY |
99% min |
|
MELTING POINT |
223 ~ 225 C |
| OPTICAL ROTATION |
-19° ~ -21° |
|
LOSS ON DRYING |
1% max |
|
HEAVY METALS |
10ppm max |
|
|
| TRANSPORT & REGULATORY INFORMATION | |
|
UN NO. |
|
| HAZARD CLASS |
|
| PACKING GROUP | |
|
|
| SAFETY INFORMATION | |
|
HAZARD OVERVIEW |
GHS (Globally Harmonised System) Classification: Acute toxicity (Oral). Acute toxicity (Inhalation). Acute toxicity (Dermal). Hazard statements: Harmful if swallowed or in contact with skin. Harmful if inhaled. |
| SIGNAL WORD | Warning |
|
PICTOGRAMS |
|
|
HAZARD STATEMENTS |
H302 + H312-H332 |
|
P STATEMENTS |
P261-P264-P270-P271-P280-P301 + P312-P302 + P352-P304 + P340-P312-P322-P330-P363-P501 |
| EC DIRECTIVES |
|
| HAZARD CODES |
|
|
RISK PHRASES |
20/21/22 |
|
SAFETY PHRASES |
22-36 |
|
|
| PACKING |
|
Preserve in light-resistant and well-closed containers |
|
|