|
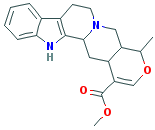
RAUBASINE |
| Synonyms. Raubasine; 16,17-Didehydro-19-methyloxayohimban-16-carboxylic acid methyl ester; Ajmalicin; Alkaloid C; Circolene; delta-Yohimbine; Hydrosarpan; Lamuran; Methyl (19-methyl-16,17-dehydro-18-oxa-3alpha,15alpha,19beta,20beta-yohimban-16-carboxylat); Methyl 16,17-didehydro-19alpha-methyl-18-oxayohimban-16-carboxylat; py-Tetrahydroserpentine; Ranitol; Raubaserp; Raubasil; Raubasin; Raubasine; Raumalina; Rauvasan; Sarpan; Substance II; Tensyl; Tetrahydroserpentine; Vincain; Vincein; Vinceine; |
|
|
| PRODUCT IDENTIFICATION | |
|
CAS RN |
483-04-5 (net), 4373-34-6 (HCl) |
|
EINECS RN |
207-589-5 (net), 224-471-9 (HCl) |
|
FORMULA |
C21H24N2O3 |
|
MOLE WEIGHT |
352.43 |
|
H.S CODE |
2939.99.0000 |
|
SMILES |
C(OC)(=O)C1=CO[C@H]([C@@H]2[C@@H]1C[C@@H]1[N@@](C2)CCc2c 3ccccc 3[nH]c12)C |
|
CLASSIFICATION |
Indole alkaloid, Antihypertensive agent |
|
EXTRA NOTES |
Ajmalicine is structurally related to yohimbine, rauwolscine, and other yohimban derivatives. Like corynanthine, it acts as a α1-adrenergic receptor antagonist with preferential actions over α2-adrenergic receptors, underlying its hypotensive rather than hypertensive effects. |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES | |
|
PHYSICAL STATE. |
white to off-white crystalline powder |
|
MELTING POINT |
258 C |
|
BOILING POINT |
|
|
DENSITY |
|
|
SOLUBILITY IN WATER |
|
| SOLVENT SOLUBILITY |
|
|
VAPOR DENSITY |
|
|
log P(octanol-water) |
2.23 |
|
VAPOR PRESSURE |
|
|
AUTOIGNITION TEMP |
|
| pK |
|
|
REFRACTIVE INDEX |
|
|
FLASH POINT |
|
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions. |
|
INCOMPATIBLE MATERIALS |
Strong oxidizing agents |
| POLYMERIZATION |
Has not been reported |
|
NFPA RATINGS |
Health: 2, Flammability: 0, Reactivity: 0 |
|
|
| EXTERNAL LINKS & GENERAL DESCRIPTION |
|
USA.gov - Raubasine Wikipedia Linking - Ajmalicine Google Scholar Search - Raubasine U.S. National Library of Medicine - Raubasine PubChem Compound Summary - Raubasine KEGG (Kyoto Encyclopedia of Genes and Genomes) - Raubasine ChEMBL (http://www.ebi.ac.uk/) - Raubasine NCBI (http://www.ncbi.nlm.nih.gov/) - Raubasine Metabolite in the indole alkaloid biosynthesis (serpentine production); found naturally in various plants such as Rauwolfia spp., Catharanthus roseus, and Mitragyna speciosa. It shows antimicrobial activity, and is used as an anti-hypertensive and sedative. sigmaaldrich |
|
|
| SALES SPECIFICATION | |
|
APPEARANCE |
white to off-white crystalline powder |
|
ASSAY |
98% min |
|
MELTING POINT |
256 ~ 258 C |
| OPTICAL ROTATION |
+64° ~ +66° (c=1, Chloroform) |
|
LOSS ON DRYING |
1% max |
|
HEAVY METALS |
10ppm max |
|
|
| TRANSPORT & REGULATORY INFORMATION | |
|
UN NO. |
|
| HAZARD CLASS |
|
| PACKING GROUP | |
|
|
| SAFETY INFORMATION | |
|
HAZARD OVERVIEW |
GHS (Globally Harmonised System) Classification: Acute toxicity (Oral). Hazard statements: Harmful if swallowed. |
| SIGNAL WORD | Warning |
|
PICTOGRAMS |
|
|
HAZARD STATEMENTS |
H302 |
|
P STATEMENTS |
|
| EC DIRECTIVES |
|
| HAZARD CODES |
|
|
RISK PHRASES |
22 |
|
SAFETY PHRASES |
|
|
|
| PACKING |
|
Preserve in light-resistant and well-closed containers |
|
|