|
ROTTLERIN |
| Synonyms. Rottlerin; Kamalin; Mallotoxin; (E)-1-(6-((3-Acetyl-2,4,6- trihydroxy-5-methylphenyl)methyl)- 5,7- dihydroxy-2,2-dimethyl-2H-1-benzopyran -8-yl)-3-phenyl-2-propen- 1-one; 3'-((8-Cinnamoyl-5,7- dihydroxy- 2,2-dimethyl-2H-1-benzopyran-6-yl) methyl)-2',4',6'-trihydroxy- 5'-methylacetophenone; |
|
|
| PRODUCT IDENTIFICATION | |
|
CAS RN |
82-08-6 |
|
EINECS RN |
201-395-4 |
|
FORMULA |
C30H28O8 |
|
MOLE WEIGHT |
516.54 |
|
H.S CODE |
2932.99.6100 |
|
SMILES |
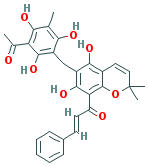
c1(c2c(c(c(c1O)Cc1c(c(c(c(c1O)C)O)C(=O)C)O)O)C=CC(O2)(C)C) C(=O)/ C= C/c1ccccc1 |
|
CLASSIFICATION |
Phloroglucinol |
|
EXTRA NOTES |
A chromenol that is 2,2-dimethyl-2H-chromene substituted by hydroxy groups at positions 5 and 7, a 3-acetyl-2,4,6-trihydroxy-5-methylbenzyl group at position 6 and a (1E)-3-oxo-1-phenylprop-1-en-3-yl group at position 8. A potassium channel opener, it is isolated form Mallotus philippensis. ChEBI |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES | |
|
PHYSICAL STATE |
white to off-white crystalline powder |
|
MELTING POINT |
212 C |
|
BOILING POINT |
|
|
DENSITY |
|
|
SOLUBILITY IN WATER |
|
| SOLVENT SOLUBILITY |
|
|
VAPOR DENSITY |
7.5 |
|
log P(octanol-water) |
|
|
VAPOR PRESSURE |
|
|
AUTOIGNITION TEMP |
|
| pK |
|
|
REFRACTIVE INDEX |
|
|
FLASH POINT |
|
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions. |
|
INCOMPATIBLE MATERIALS |
Strong oxidizing agents. |
| POLYMERIZATION |
Has not been reported |
|
NFPA RATINGS |
Health: 1, Flammability: 1, Reactivity: 0 |
|
|
| EXTERNAL LINKS & GENERAL DESCRIPTION |
|
USA.gov - Rottlerin Wikipedia Linking - Rottlerin Google Scholar Search - Rottlerin U.S. National Library of Medicine - Rottlerin PubChem Compound Summary - Rottlerin KEGG (Kyoto Encyclopedia of Genes and Genomes) - Rottlerin ChEBI (http://www.ebi.ac.uk/chebi/) - Rottlerin NCBI (http://www.ncbi.nlm.nih.gov/) - Rottlerin Material Safety Data Sheet - Rottlerin Recently, Rottlerin (mallotoxin) has been shown to be a potent activator of the large conductance voltage and Ca2 activated K+ channel and to potently leftward shift the conductance-voltage relationship of the channel. Mallatoxin tested on hERG channels increased both step and tail hERG current by leftward shifting the voltage dependence of hERG activation and slowing channel deactivation. These actions distinguish Mallatoxin as a novel naturally occurring hERG channel activator. sigmaaldrich |
|
|
| SALES SPECIFICATION | |
|
APPEARANCE |
white to off-white crystalline powder |
|
ASSAY |
98% min |
|
MELTING POINT |
212 C |
|
HEAVY METALS |
10ppm max |
|
|
| TRANSPORT & REGULATORY INFORMATION | |
|
UN NO. |
|
| HAZARD CLASS |
|
| PACKING GROUP | |
|
|
| SAFETY INFORMATION | |
|
HAZARD OVERVIEW |
GHS (Globally Harmonised System) Classification: Not a dangerous substance. |
|
|
| PACKING |
|
Preserved in light-resistant and well-closed bottles |
|
|