|
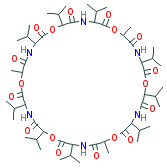
VALINOMYCIN |
| Synonyms. Valinomycin; 12,24,36-trimetyl-3,6,9,15,18,21,27,30,33-Nonakis(1-methylethyl)-1, 7,13,19,25, 31-hexaoxa-4,10,16,22,28,34-hexaazacyclohexatriacontane-2 ,5,8,11,14,17,20,23,26,29,32, 35-dodecone; Cyclic(D-alpha-hydroxyisovaleryl-D-valyl-L- lactoyl-L-valyl-D-alpha-hydroxyisovaleryl-D- valyl-L- lactoyl-L-valyl- D-alpha-hydroxyisovaleryl-D-valyl-L- lactoyl-L-valyl); Valinomicin; Cyclo(L-Val-D-HyIva-D-Val-L-Lac-)3 (HyIva = akoha-Hydroxyisovaleric acid, Lac = Lactic acid); |
|
|
| PRODUCT IDENTIFICATION | |
|
CAS RN |
2001-95-8 |
|
EINECS RN |
217-896-6 |
|
FORMULA |
C54H90N6O18 |
|
MOLE WEIGHT |
1111.32 |
|
H.S CODE |
2941.90.1010 |
|
SMILES |
C1([C@@H](OC([C@@H](NC([C@@H](OC([C@@H](NC([C@@H] (OC([C @@H](NC(=O)[C@@H](OC([C@@H](NC([C@@H](OC([C@@H] (NC([C@@ H]( OC([C@@H](N1)C(C)C)=O)C)= O)C(C)C)=O)C(C)C)=O)C(C)C)=O) C)C(C)C)= O)C(C)C)=O)C (C)C)=O)C)=O)C(C)C)= O)C(C)C)=O; |
|
CLASSIFICATION |
Antibacterial, Anti-Infective, Ionophore, Cyclic peptide, Depsipeptide |
|
EXTRA NOTES |
A cyclododecadepsipeptide ionophore antibiotic produced by Streptomyces fulvissimus and related to the enniatins. It is composed of 3 moles each of L-valine, D-alpha-hydroxyisovaleric acid, D-valine, and L-lactic acid linked alternately to form a 36-membered ring. (From Merck Index, 11th ed) Valinomycin is a potassium selective ionophore and is commonly used as a tool in biochemical studies [MeSH] |
|
|
| PHYSICAL AND CHEMICAL PROPERTIES | |
|
PHYSICAL STATE. |
white to off-white crystalline powder |
|
MELTING POINT |
187 ~ 190 C |
|
BOILING POINT |
|
|
DENSITY |
|
|
SOLUBILITY IN WATER |
Insoluble |
| SOLVENT SOLUBILITY |
|
|
VAPOR DENSITY |
|
|
log P(octanol-water) |
4.49 |
|
VAPOR PRESSURE |
|
|
AUTOIGNITION TEMP |
|
| pK |
|
|
REFRACTIVE INDEX |
|
|
FLASH POINT |
|
|
|
| STABILITY AND REACTIVITY | |
| STABILITY | Stable under normal conditions. |
|
INCOMPATIBLE MATERIALS |
Strong oxidizing agents |
| POLYMERIZATION |
Has not been reported |
|
NFPA RATINGS |
Health: 4, Flammability: 0, Reactivity: 0 |
|
|
| EXTERNAL LINKS & GENERAL DESCRIPTION |
|
USA.gov - Valinomycin Wikipedia Linking - Valinomycin Google Scholar Search - Valinomycin U.S. National Library of Medicine - Valinomycin PubChem Compound Summary - Valinomycin KEGG (Kyoto Encyclopedia of Genes and Genomes) - Valinomycin http://www.ebi.ac.uk/chebi/ - Valinomycin http://www.ncbi.nlm.nih.gov/ - Valinomycin Hazardous Substances Data Bank - Valinomycin EPA - Substance Registry Services - Valinomycin http://www.sigmaaldrich.com/ |
|
|
| SALES SPECIFICATION | |
|
APPEARANCE |
white crystalline powder |
|
ASSAY |
95% min |
|
MELTING POINT |
180 ~ 185 C |
| OPTICAL ROTATION |
+30° ~ +32° (c=1.6, Benzene) |
|
WATER |
0.5% max |
|
HEAVY METALS |
10ppm max |
|
|
| TRANSPORT & REGULATORY INFORMATION | |
|
UN NO. |
2811 |
| HAZARD CLASS |
6.1 |
| PACKING GROUP | I |
|
|
| SAFETY INFORMATION | |
|
HAZARD OVERVIEW |
GHS (Globally Harmonised System) Classification: Acute toxicity (Oral), Acute toxicity (Dermal). Hazard statements: Fatal if swallowed or in contact with skin. Potential Health Effects: Eyes - May cause eye irritation. Skin - May be fatal if absorbed through skin. May cause skin irritation. Ingestion - May be fatal if swallowed. Inhalation - May be harmful if inhaled. May cause respiratory tract irritation. |
| SIGNAL WORD | Danger |
|
PICTOGRAMS |
|
|
HAZARD STATEMENTS |
H300+H310 |
|
P STATEMENTS |
P264-P280-P301 + P310-P302 + P350-P310 |
| EC DIRECTIVES |
|
| HAZARD CODES |
|
|
RISK PHRASES |
27/28 |
|
SAFETY PHRASES |
28-36/37-45 |
|
|
| PACKING |
|
Preserved in light-resistant and well-closed bottles |
|
|