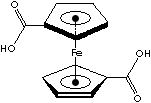
| 1,1'-DICARBOXYFERROCENE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 1293-87-4 |
|
| EINECS NO. |
215-068-9 | |
| FORMULA |
C12H10FeO4 | |
| MOL WT. |
274.05 | |
| H.S. CODE | ||
|
TOXICITY |
||
| SYNONYMS | Bis(carboxycyclopentadienyl)iron; 1,1'-Ferrocenedicarboxylic acid; | |
|
DERIVATION |
|
|
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | dark orange powder | |
| MELTING POINT |
300 C | |
| BOILING POINT |
| |
| SPECIFIC GRAVITY |
| |
| SOLUBILITY IN WATER | Insoluble | |
|
SOLVENT SOLUBILITY |
||
| pH | ||
| VAPOR DENSITY | ||
| AUTOIGNITION |
| |
| NFPA RATINGS | Health: 1; Flammability: 1; Reactivity: 0 | |
| REFRACTIVE INDEX |
||
| FLASH POINT | ||
| STABILITY | Stable under ordinary conditions | |
|
DESCRIPTION AND APPLICATIONS |
||
Ferrocene, yellow to orange crystal melting at 173 C, is an organometallic
compound of sandwich structure. It has two five membered carbon rings which
are
parallel, with the iron ion sandwiched between them. Its systematic name is
Di-[��]-Cyclopentadienyl Iron(II) or Bis(��5-Cyclopentadienyl) Iron. The bonding is between pi orbitals on the
rings and d-orbitals on the Fe2+ ion. It is used as a combustion control
additive in fuels, antiknock agent in gasoline,
and for heat stabilization in greases and plastics. It is used as a catalyst for the synthesis of ammonia. Ferrocene derivatives, can be described as a multi-electron transfer, are useful
for following fields:
|
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
dark orange powder | |
|
CONTENT |
96.0% min | |
|
FREE-Fe |
100ppm max | |
| TRANSPORTATION | ||
| PACKING | ||
| HAZARD CLASS | ||
| UN NO. | ||
| OTHER INFORMATION | ||
| Hazard Symbols: XI, Risk Phrases: 20/21/22, Safety Phrases: 36 | ||
|
GENERAL DESCRIPTION OF METALLOCENE |
||
| Metallocene is a compound consisting of parallel ring system ligand bound to a metal. Ligand, in organometallic chemistry, is a molecule that donates or accepts a pair of electrons to form a coordinate covalent bond with the central metal atom of a coordination complexes and organometallic compounds; can be further distinguished by the orbitals used in bond formation. There can be numerous organometallic coordination compounds distinguished by number of cyclopentadienyl rings (with or without substituents) bonded to a central transition-metal atoms and the types of transition-metals and the type of the bridge. Metals known to form metallocene complexes are titanium, zirconium, hafnium, vanadium, chromium, molybdenum, tungsten, manganese, iron, ruthenium, osmium, cobalt, rhodium, and nickel. Metallocenes possess the transition-metal atoms whose bonding involves overlap of ns, (n - 1)d, and np orbitals of the metal with molecular orbitals of appropriate symmetry of each aromatic (bis-cyclopentadienyl) rings. The commonest metallocene is ferrocene, yellow to orange crystal melting at 173 C, is an organometallic compound of sandwich structure. It has two five membered carbon rings which are parallel, with the iron ion sandwiched between them. The equivalent bonding of all 5 carbon atoms of each cyclopentadienyl ring in ferrocene is denoted as eta-5. Its systematic name is Di-[��]-Cyclopentadienyl Iron(II) or Bis(��5-Cyclopentadienyl) Iron. The bonding is between pi orbitals on the rings and d-orbitals on the Fe2+ ion. It is used as a combustion control additive in fuels, antiknock agent in gasoline, and for heat stabilization in greases and plastics. It is used as a catalyst for the synthesis of ammonia. Metallocenes having catalytic site in systematic molecular structure are used as polymerization catalyst to produce uniform polymer with unique structures and physical properties. Intensive study of metal-carbon bonds have been developed in the field of electrochemical techniques, high-temperature chemistry, photolysis chemistry, structural chemistry, organic light-emitting devices, biochemistry and pharmaceutical manufacturing. | ||