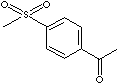
| 4'-METHYLSULFONYLACETOPHENONE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 10297-73-1 |
|
| EINECS NO. | 233-672-0 | |
| FORMULA | H3CC(O)C6H4SO2CH3 | |
| MOL WT. | 198.24 | |
| H.S. CODE | ||
|
TOXICITY |
||
| SYNONYMS | 1-[4-(Méthylsulfonyl)phényl]éthane-1-one; | |
| 1-[4-(methylsulphonyl)phenyl]ethan-1-one; 1-[4-(metilsulfonil)fenil]etan-1-ona; 4-Methylsulphonylacetophenone; | ||
|
DERIVATION |
|
|
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | white to off-white crystals | |
| MELTING POINT | 126 - 131 C | |
| BOILING POINT | ||
| SPECIFIC GRAVITY | ||
| SOLUBILITY IN WATER | ||
| pH |
|
|
| VAPOR DENSITY | ||
| AUTOIGNITION |
|
|
| NFPA RATINGS | ||
|
REFRACTIVE INDEX |
||
| FLASH POINT | ||
| STABILITY | Stable under ordinary conditions. | |
|
APPLICATIONS |
||
|
Acetophenone, the simplest aromatic ketone, is a clear liquid or crystals;
melting point 19 - 20 C; boiling point 202 C; very slightly soluble in water.
Commercial acetophenone can be obtained from benzene with
acetic anhydride or acetyl chloride by Friedel-Crafts process.
It can be also obtained by air oxidation
of
ethylbenzene, as a by-product of cumene
or from acrylonitrile. It
is used as a polymerization catalyst for the manufacture of olefins. It is used
as an intermediate for pharmaceuticals, agrochemicals and other organic
compounds. It also has been used as a drug to induce sleep. Its is used in tear
gas (especially as the form of chloro acetophenone) and warfare. It is used as
an solvent for plastics, resins, cellulose ethers, and esters. The dimer
(dipnone) is used as a plasticizer. Actophenone and its derivatives, having
additionally substituted saturated alkyls, oxygenated alkyl groups, thio groups,
additional aromatic groups, unsaturated aliphatic side chains, and other
functional groups, are ingredients of flavor & fragrance for in soaps,
detergents, cosmetics, and perfumes as well as in foods, beverages, and tobacco.
Mercaptan: any of a class of organosulfur compounds is similar to the alcohol and phenol but containing a sulfur atom in place of the oxygen atom. Compounds containing -SH as the principal group directly attached to carbon are named 'thiols'. In substitutive nomenclature their names are formed by adding '-thiol' as a suffix to the name of the parent compound. When -SH is not the principal group, the prefix 'mercapto-' is placed before the name of the parent compound to denote an unsubstituted -SH group. 'thio' is a chemical prefix indicates the replacement of an oxygen in an acid radical by sulfur with a negative valence of 2. Sulfur analog of alcohol is called thiol (or mercaptan), and ether analog is called sulfide. The first chemical contrast of thiols and sulfides with alcohols and ethers is acidity which is important in organic reactions. Thiols are stronger acids than relevant alcohols and phenols. Thiolate conjugate bases are easily formed, and are excellent nucleophiles in SN2 reactions of alkyl halides and tosylates. The nucleophilicity of sulfur is much greater than that of oxygen, resulting in a number of useful electrophilic substitution reaction that are rare by oxygen. For example, sulfides form (with alkyl halides) ternary sulfonium salts, in the same alkylattion of tert-amines quaternary ammonium salts, whereas ternary oxonium salts are prepared only under extream conditions. Without exception, sulfoxides, sulfinate salts and sulfite anion also alkylate on sulfur, despite of the partial negative formal charge on oxygen and partial positive charge on sulfur. The second character is the oxidation states of sulfur. Oxygen has only two oxidation states, whereas sulfur covers from –2 to +6 as follows:
One more sulfur compound's contrast with oxygen analog is in oxidation chemistry. Oxidation of sulfur compounds changes the oxidation state of sulfur rather than carbon, whereas, oxidation of alcohols to aldehydes and ketones changes the oxidation state of carbon not oxygen. Thiols is oxidized to S-S single bond (disufide) which is stronger than O–O bond in peroxide. Disufide forms sulfenyl chlorides (with chlorine in mild condition) or sulfonic acids under harder condition. Oxidation of sulfides with hydrogen peroxide (or peracids) yields sulfoxides and then to sulfones. A certain sulfoxide compound such as dimethyl sulfoxide can be used as an effective oxygen source in the oxidation reaction of primary and secondary alcohols to aldehydes and ketones. DMSO easily is reduced to dimethyl sulfide and water is taken up by the electrophile. oxidation procedure is very mild and tolerates a variety of other functional groups, including those having oxidizable nitrogen and sulfur atoms. |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
white to off-white crystals | |
|
ASSAY |
95.0% min |
|
|
MELTING POINT |
126 - 131 C |
|
| TRANSPORTATION | ||
| PACKING |
|
|
| HAZARD CLASS | ||
| UN NO. | ||
| OTHER INFORMATION | ||
| Hazard Symbols: , Risk Phrases: , Safety Phrases: 24/25 | ||