| SALICYLHYDRAZIDE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 936-02-7 |
|
| EINECS NO. | 213-311-3 | |
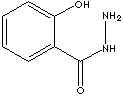
| FORMULA | (HO)C6H4CONHNH2 | |
| MOL WT. | 152.15 | |
| H.S. CODE | ||
|
TOXICITY |
|
|
| SYNONYMS | Salicylic acid hydrazide; o-Hydroxybenzoylhydrazine; | |
| 2-Hydroxybenzhydrazide; 2-Hydroxy-benzoic acid hydrazide; | ||
| DERIVATION |
|
|
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | white to off-white crystalline powder | |
| MELTING POINT |
147 - 150 C |
|
| BOILING POINT | ||
| SPECIFIC GRAVITY | ||
| SOLUBILITY IN WATER |
|
|
| AUTOIGNITION |
|
|
| VISCOSITY | ||
| PH |
|
|
| VAPOR DENSITY | ||
| NFPA RATINGS | Health: 2; Flammability: 0; Reactivity: 0 | |
| FLASH POINT | ||
| STABILITY | Stable under ordinary conditions | |
|
GENERAL DESCRIPTION & APPLICATIONS |
||
|
Salicylic Acid
is a white crystalline powder or needle-shaped crystals with sweetish taste;
soluble in acetone, ether, alcohol, boiling water, benzene and turpentine,
sparingly soluble in chloroformbenzene, slightly soluble in water; melts at
158°C. The sodium salt form (sodium salicylate) is common commercially, prepared
from mainly sodium phenolate with carbon dioxide under heating and pressure. It
contains both a hydroxyl and a carboxyl group, which react with either an acid
or an alcohol. The carboxyl group forms esters with alcohols; e.g. methyl
salicylate is formed with methanol, which used in food flavorings and
preservatives; menthyl salicylate is formed with methanol, which is used in
suntan lotions. The hydroxyl group reacts with acetic acid to form
acetylsalicylic acid (called aspirin) which is the most widely common antiseptic
and antipyretic agent. Phenyl salicylate (called salol) is formed with phenol,
which is also used as an antiseptic and antipyretic agent. The sodium salt
(Sodium salicylate), a shiny white powder, is used for antiseptics preparations
and as a preservative. In addition to its analgesic and antipyretic properties,
salicylic acid possesses keratinolytic properties and fungicidal properties. It
ans its derivatives are used in the treatment of hyperkeratotic, dandruff,
ichthyosis and psoriasis as well as in the treatment of fungal skin infections
such as tinea. Para-Aminosalicylic acid (abbreviated PAS and PASA) is an
analogue of para-aminobenzoic acid (abbreviated PABA) that inhibits folic acid
synthesis in Mycobacterium tuberculosis and is bacteriostatic, inhibits growth
and multiplication of the tubercle bacillus. Para-Aminosalicylic acid and its
sodium salt (sodium p-Aminosalicylate) are bacteriostatic against mycobacteria
and used in the treatment of tuberculosis; administered orally. Brand names are
Tubasal, Nemasol Sodium and etc. Aminosalicylic acids are pharmaceutically
active ingredients including anti-infectives against colds, flu, or other virus
infections. Mesalamine (5-aminosalicylic acid, abbreviated 5-ASA) an active
metabolite of sulfasalazine, used to treat inflammation of the rectum and lower
colon, mild to moderate ulcerative colitis proctosigmoiditis, and proctitis.
Para-Aminosalicylic acid (4-hydroxybenzoic acid) is used as an intermediate of
bacteriostatic agent specially for
parabens (alkyl esters of p-hydroxy benzoic acid)
which used in food and personal care products as a preservative. It is applied in the
production of liquid crystal polymers. It is also used as an
intermediate of dyes, insecticides, pharmaceutical, pesticides and other
chemical compounds. Salicylic Acid and its derivatives are important
for the preparation of other pharmaceutical products, dyes, flavours, and
preservatives.
Hydrazine (anhydrous) is a clear, fuming, corrosive liquid with an ammonia-like odor; melting at 1.4 C, boiling at 113.5 C, specific gravity 1.011. It is very soluble in water and soluble in alcohol. It decomposes on heating or exposure to UV to form ammonia, hydrogen, and nitrogen, which may be explosive with a blue flame when catalysed by metal oxides and some metals such as platinum or Raney nickel. It is prepared from ammonia with chloramine in the presence of glue or gelatin (to inhibit decomposition of the hydrazine by unreacted oxidants) as the hydrate form usually (100% monohydrate contains 64% by weight hydrazine). Hydrazine is also prepared from sodium hypochlorite with urea in the presence of glue or gelatin. Botht ammonia and amines are nitrogen nucleophiles which donate electrons (they are Lewis bases). But hydrazine (diamine) has much stronger nucleophilicity which makes it more reactive than ammonia. Hydrazine has dibasic and very reactive properties. Hydrazine is used as a component in jet fuels because it produce a large amount of heat when burned. Hydrazine is used as rocket fuel. Hydrazine is used as an oxygen scavenger for water boiler feed and heating systems to prevent corrosion damage. Hydrazine is used as a reducing agent for the recovery of precious metals. It is used as a polymerization catalyst and a chain extender in urethane coatings. It and its derivatives are versatile intermediates. They have active applications in organic synthesis for agrochemicals, pharmaceuticals, photographic, heat stabilizers, polymerization catalysts, flame-retardants, blowing agents for plastics, explosives, and dyes. Recently, hydrazine is applied to LCD (liquid crystal displays) as the fuel to make faster thin-film transistors. Hydrazide is an acyl hydrazine. Acyl (-CO) is an organic radical formed by removal of a hydroxyl group from an organic acid (carboxyl group). |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
white to off-white crystalline powder | |
| ASSAY |
98.0% min |
|
| TRANSPORTATION | ||
| PACKING | ||
| HAZARD CLASS | ||
| UN NO. | ||
| OTHER INFORMATION | ||
|
Hazard Symbols: XI, Risk Phrases: 36/37/38, Safety Phrases: 23-24/25 |
||