|
BORIC ACID
|
||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. |
10043-35-3,
11113-50-1 (Base) 13813-79-1 (orthoboric acid) 13460-50-9, 13780-71-7 (metaboric acid) |
|
| EINECS NO. | 233-139-2,
234-343-4 (Base) 237-478-7 (orthoboric acid) 236-659-8, 237-432-6 (metaboric acid) |
|
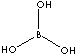
| FORMULA | H3BO3 or B2O3·3H2O | |
| MOL WT. | 61.83 | |
|
H.S. CODE |
2810.00 | |
| TOXICITY | Oral rat LD50: 2660 mg/kg | |
| SYNONYMS | Boracic Acid, Hydrogen Borate, Orthoboric Acid; | |
| Boracic acid; Hydrogen orthoborate; Trihydroxyborane; Borsäure (German); ácido bórico (Spanish); Acide borique (French); | ||
| SMILES | ||
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | white cystals | |
| MELTING POINT |
170 C |
|
| BOILING POINT | 300 C | |
| SPECIFIC GRAVITY | 1.43 - 1.44 | |
| SOLUBILITY IN WATER | 4 - 5 g/100 ml at 20 C | |
| pH | 5.2 (1% sol.) | |
| VAPOR DENSITY | ||
|
AUTOIGNITION |
|
|
|
NFPA RATINGS |
Health: 2; Flammability: 0; Reactivity: 0 | |
|
REFRACTIVE INDEX |
|
|
| FLASH POINT | ||
| STABILITY | Stable under ordinary conditions. Hygroscopic. | |
|
GENERAL DESCRIPTION AND APPLICATIONS |
||
| Boric acid refers to 3 compounds; orthoboric acid (also called boracic acid, H3BO3 or B2O3·3H2O), metaboric acid (HBO2 or B2O3·H2O), and tetraboric acid (also called pyroboric, H4B4O7 or B2O3·H2O). Orthoboric acid dehydrates to form metaboric acid and tetraboric acid above 170 C and 300C respectively. Orthoboric acid is derived from boric oxide in the form of white, triclinic crystals. It is poorly soluble in cold water but dissolves readily in hot water, in alcohol and glycerine. Metaboric acid is a white, cubic crystalls. It is soluble in water slightly. Tetraboric acid is a white solid soluble in water. When tetraboric and metaboric acid are dissolved, it reverts to orthoboric acid. The main uses of boric acid is to make borate salts such as borax and other boron compounds. Boric acid is also used in heat resistant glass, in fireproofing fabrics, in electroplating baths, in leather manufacturing, porcelain enamels and in hardening steels. Boric acid has antiseptic and antiviral activity. Aqueous solutions have been used as mouth-washes, eye-drops, skin lotions and cosmetics. Boric acid and its salts are components of many commercial insecticides and wood preservatives. | ||
| SALES SPECIFICATION | ||
|
APPEARANCE |
white cystals |
|
| BH3O3 |
99.0% min |
|
| PHOSPHATES |
0.001% max |
|
|
SULFATES |
0.005% max |
|
| INSOLUBLES | 0.005% (in methanol) | |
|
Ca |
50ppm max |
|
|
Cl |
10ppm max |
|
|
As |
1ppm max |
|
|
HEAVY METALS (as Pb) |
10ppm max |
|
|
TRANSPORTATION |
||
| PACKING | 25kgs in bag | |
| HAZARD CLASS | ||
| UN NO. | ||
|
OTHER INFORMATION |
||
| Hazard Symbols: T, Risk Phrases: 60, Safety Phrases: 53-45 | ||
|
GENERAL DESCRIPTION OF BORON AND ITS COMPOUNDS |
||
|
Boron is a nonmetallic element, group III in the periodic table. Symbol B; aomic
number 5; atomic mass 10.811; melting point ca 2,300 C; sublimation point ca
2,550 C; specific gravity 2.37 or 2.34; valence +3; electronic config.
[He]2s22p1. There are two allotropes of boron; amorphous boron is a dark brown
to black amorphous powder, but metal-like crystalline solid is an extremely hard
(9.3 on Mohs' scale), black to silver-gray, brittle, lustrous and has a bad
conductor in room temperatures. The specific gravities of amorphous and
crystalline forms are 2.37 and 2.34 respectively. The crystalline form is far
less reactive than the amorphous form. The amorphous powder is oxidized slowly
in air at room temperature and ignites spontaneously at high temperatures to
form an oxide but the crystalline form is oxidized only very slowly, even at
higher temperatures. Boron is widely distributed in the form of borates but is
never found in the elemental form in nature. The important commercial borate
products are borax penta (or deca) hydrate, boron oxide, sodium perborate, boric
acid and minerals are borax, colemanite, ulexite, tincal, kermite, and brines as
well as ascharite, hydroboracite, datolite, tourmaline, etc. The simple way to
prepare boron of amorphous powder form is the reduction of boron trioxide by
heating with magnesium. Boric acid is produced mainly from borate ores
containing sodium or calcium by the reaction with sulfuric acid in the presence
of a hot aqueous boric acid liquor to recycle.
Major end uses for borates include;
|
||