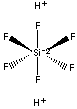|
FLUOROSILICIC ACID
| ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 16961-83-4; 1309-45-1; 12672-67-2 |
|
| EINECS NO. | 241-034-8 | |
| FORMULA |
H2SiF6 | |
| MOL WT. | 144.08 | |
|
H.S.CODE |
2811.19.6090 | |
| TOXICITY | Rat LD50 (Oral): 430mg/kg | |
| SYNONYMS |
Hydrofluorosilicic Acid; Hydrosilicofluoric acid; | |
|
Sand acid; Silicofluoric acid; Fluosilicic acid; Hydrofluorosilicic acid; Hydrofluosilic Acid; Hexafluorosilicic acid; Dihydrogen hexafluorosilicate; 氟硅酸; Hexafluorokieselsäure (Dutch); ácido hexafluorosilicico (Spanish); Acide hexafluorosilicique (French); Silicofluoric acid; Silicofluoride; Silicon hexafluoride dihydride; Fluorosilicic acid; H2SiF6; Hydrogen hexafluorosilicate; |
||
| SMILES | by-product of phosphate fertilizer or HF and silica | |
|
SMILES |
[F-][Si+4]([F-])([F-])([F-])([F-])[F-] | |
|
CLASSIFICATION |
Hydrogen compound, Fluoride, Silicate, Acid, Coordination compound |
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | white to yellow solution | |
| MELTING POINT | -15.5 C | |
| BOILING POINT | 105 C | |
| SPECIFIC GRAVITY | 1.46 (60%), 1.28 ( 30%) | |
| SOLUBILITY IN WATER | Complete | |
| pH | 1.2 (1% Sol.) | |
| VAPOR DENSITY | ||
|
AUTOIGNITION |
| |
|
NFPA RATINGS |
| |
|
REFRACTIVE INDEX |
| |
| FLASH POINT |
| |
| STABILITY | Stable under ordinary conditions | |
|
GENERAL DESCRIPTION & EXTERNAL LINKS |
||
|
Water
fluoridation, Wood preservative, Sterilization of
equipment, Electroplating, Tanning, Glass
etching, Cement Hardening, Oil
acidizing, Rust Removal in textile field, lead
refining and fluorosilicate salt manufacture. Wikipedia Linking:http://en.wikipedia.org/wiki/Hexafluorosilicic_acid http://www.fluoridealert.org/ http://www.cdc.gov/niosh/ipcsneng/neng1233.html http://www.solvaychemicals.us/ |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
white to yellow solution | |
| ASSAY (H2SiF6) |
23.0% min | |
| FLUORINE (F) |
18.0% min | |
| HEAVY METALS |
20ppm max | |
| HYDROFLUORIC ACID (HF) |
1.0% max | |
| VISCOSITY |
6.5 cps | |
| TRANSPORTATION | ||
| PACKING | 25kgs in plastic drum | |
| HAZARD CLASS | 8 (Corrosive) | |
| UN NO. |
1778 (Packing group: II) | |
| OTHER INFORMATION | ||
|
-Fluorosilicic
Acid is available generally as a 20 to 40 percent aqueous solution. |
||
|
PRICES |
||