| POTASSIUM CHLORATE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 3811-04-9 |
|
| EINECS NO. | 223-289-7 | |
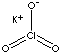
| FORMULA | KClO3 | |
| MOL WT. | 122.54 | |
|
H.S. CODE |
2829.11 | |
| TOXICITY | Oral rat LD50: 1870 mg/kg | |
| SYNONYMS | Potash Chlorate; Chloric Acid, Potassium Salt; | |
| Berthollet salt; Chlorate of Potash; | ||
| SMILES | electrolysis of hot concentrated solutions of potassium chloride | |
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | white crystalline solid, saline taste | |
| MELTING POINT |
356 C |
|
| BOILING POINT | Decomposes above 400 C | |
| SPECIFIC GRAVITY | 2.32 | |
| SOLUBILITY IN WATER | soluble (moderately soluble in ethanol) | |
| pH | ||
| VAPOR DENSITY | 4.2 | |
|
AUTOIGNITION |
||
|
NFPA RATINGS |
Health: 1 Flammability: 0 Reactivity: 1 Other: Oxidizer | |
|
REFRACTIVE INDEX |
|
|
| FLASH POINT | Not combustible | |
| STABILITY | Stable under ordinary conditions | |
|
APPLICATIONS |
||
| Potassium Chlorate is a powerful oxidizing agent. It is used to manufacture explosives and matches because of its ability to produce oxygen. Chlorate is a powerful ingredient of bleaching powder used in paper and pulp processing and calico printing. It is used also a weed killer and defoliant. | ||
| SALES SPECIFICATION | ||
|
APPEARANCE |
white crystalline solid | |
| ASSAY |
99.5%
min
|
|
|
WATER INSOLUBLES |
0.03% max |
|
| CHLORIDES |
0.02% max |
|
| SULFATE SALTS |
0.02% max |
|
|
BROMATES |
0.03% max |
|
|
Fe |
0.003% max |
|
|
WATER |
0.5% max |
|
| TRANSPORTATION | ||
| PACKING | 50kgs in drum | |
| HAZARD CLASS | 5.1 (Packing Group: II) | |
| UN NO. | 1485 | |
| OTHER INFORMATION | ||
| European Hazard Symbols: XN O, Risk Phrases: 20/22-8, Safety Phrases:13-16-27 | ||
| Chlorate is the common name for chlorate(V) salt. The related names to the acids are clear when the oxidation number statement is appended; hypochlorite [or chlorate(I)] contains the ion, ClO- ; chlorite [or chlorate(III)], ClO2-; Chlorate [or chlorate(V)], ClO3-; perchlorate [or chlorate(VII)], ClO4-. | ||