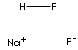|
SODIUM BIFLUORIDE |
|||
|
PRODUCT IDENTIFICATION |
|||
| CAS NO. |
1333-83-1; 7783-37-1; 17097-13-1; 70876-64-1; 51273-71-3, 7681-49-4 (Parent) |
|
|
| EINECS NO. | 215-608-3 | ||
| FORMULA |
NaHF2 |
||
| MOL WT. |
61.99 |
||
| H.S. CODE | 2826.90.9000 | ||
|
TOXICITY |
|||
| SYNONYMS | sodium hydrogen fluoride; Sodium fluoride (Na(HF2)); | ||
| Fluorure acide de soodium (French); Fluoruro acido de sodio (Spanish); Sodium Hydrogen Difluoride; Sodium Acid Fluoride; Sodium hydrogendifluoride; | |||
|
SMILES |
F.[Na+].[FH-] |
||
|
CLASSIFICATION |
Fluoride, Metal halide |
||
|
PHYSICAL AND CHEMICAL PROPERTIES |
|||
| PHYSICAL STATE |
white cristals |
||
| MELTING POINT |
160 C (Decomposes) |
||
| BOILING POINT | |||
| SPECIFIC GRAVITY | 2.08 | ||
| SOLUBILITY IN WATER | |||
| pH | 4 (1% sol.) | ||
| VAPOR DENSITY | |||
| AUTOIGNITION |
|
||
| NFPA RATINGS | |||
|
REFRACTIVE INDEX |
|||
| FLASH POINT | not flammable but HF gas is released when heated above 100 C | ||
| STABILITY | Stable under ordinary conditions | ||
|
GENERAL DESCRIPTION AND APPLICATIONS |
|||
|
Sodium Bifluoride is used as a component of electrolyte for galvanizing baths. It is used in pest control and insectproofing agent for leather. It is used in textile industry for removal of iron rust and as a neutralization agent. It can be used as a catalyst for polymerization processing. It is used for sanitation and Laundry souring agent. Wikipedia Linking: http://en.wikipedia.org/wiki/Sodium_fluoride http://www.fluoridealert.org/p http://www.pesticideinfo.org/Summary_Chemical.jsp?Rec_Id=PC35648 http://www.docep.wa.gov.au/ Hydrofluoric acid is also transported for these purposes, so may be present on trucks, trains, ships or in courier vehicles. A Poisons Permit from the Department of Health is required for purchase of solutions containing greater than 1 per cent hydrofluoric acid. |
|||
| SALES SPECIFICATION | |||
|
APPEARANCE |
White free flowing fine granular material | ||
|
NaHF2 |
99.0% min |
||
|
HF |
0.2% max |
||
|
Na2SiF6 |
0.5% max |
||
|
INSOLUBLES |
0.3% max |
||
|
HEAVY METAL |
5ppm max |
||
|
WATER |
0.2% max |
||
|
PARTICLE SIZE |
95.0% min (325 mesh) |
||
| TRANSPORTATION | |||
| PACKING |
25kgs in bag |
||
| HAZARD CLASS | 8 (Packing group : II) | ||
| UN NO. |
2439 |
||
PRICES |
|||
|
|||