SODIUM HYDROSULFITE
PRODUCT IDENTIFICATION
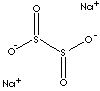
HS CODE
2831.10.5000
Oral Rat LD 50: 5 g/kg.
CLASSIFICATION
Bleach, Dithionite
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
EXTERNAL LINKS & GENERAL DESCRIPTION
USA.gov - Sodium Hydrosulfite
Wikipedia Linking - Sodium dithionite
Google Scholar Search - Sodium Hydrosulfite
U.S. National Library of Medicine - Sodium Hydrosulfite
PubChem Compound Summary - Sodium Hydrosulfite
IPCS INCHEM - Sodium Hydrosulfite
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Sodium Hydrosulfite
ChEBI (http://www.ebi.ac.uk/chebi/) - Sodium Hydrosulfite
NCBI (http://www.ncbi.nlm.nih.gov/) - Sodium Hydrosulfite
Material Safety Data Sheet - Sodium Hydrosulfite
Hazardous Substances Data Bank - Sodium Hydrosulfite
EPA - Substance Registry Services - Sodium Hydrosulfite
Local:
Sodium Hydrosulfite is a white powder. Commercial sodium hydrosulfite
contains 85% - 90% sodium dithionite w/w. It is readily soluble in water and
shows powerful reducing action in aqueous solutions. Sodium hydrosulfite is
used as a reducing agent in dying application. It undergoes reduction reaction
with water-insoluble vat dye and sulfur dye to form water-soluble alkali metal
salt of the dye (leuco form ) so that they have affinity for the textile fiber.
The reductive decomposition of the excessive dye by sodium hydrosulfite improve
the colour fastness. Sodium hydrosulfite's reduction reaction removes
residual oxide and wrong pigments. Sodium hydrosulfite is a reductive bleaching
agent. It reduces carbonyl and alcohol groups, which function as colorants of
the substances. It is used in bleaching mechanical paper pulp, cotton, wool and
kaolin clay. Additional applications include water treatment, leather
processing, food processing, gas purification, cleaning, printing and stripping.
In addition to a reducing agent function, sodium hydrosulfite functions as a
sulfonating agent and sodium ion source in a variety of chemical reactions.
SULFURIC SALTS: Sulfate (also spelled sulphate in Europe) is any chemical compound containing the SO42- ion related to sulfuric acid (H2SO4). Sulfates are salts or esters of sulfuric acid, formed by replacing one or both of the hydrogens with a metal or a radical as in sodium sulfate, Na2SO4. Sulfates in which both hydrogens are replaced are called normal sulfates. Bisulfate is a compound that has the HSO4- radical. Bisulfate (called also hydrogen sulfate or acid sulfate) is a compound formed by replacing only one hydrogen in sulfuric acid. Sulfite (also sulphite) is a compound that contain the sulfite ion SO32-. Sulfites are salts or esters of sulfurous acid (H2SO3), formed by replacing one or both of the hydrogens with a metal or a radical as in sodium sulfite, Na2SO3. Sulfites in which both hydrogens are replaced are called normal sulfites. Bisulfite is a compound that has the HSO3- radical. Bisulfate (called also hydrogen sulfite or acid sulfite) is a compound formed by replacing only one hydrogen in sulfurous acid. The term of 'meta' or 'pyro' is the chemical prefix for oxo acid formed through the loss of one water molecule (dehydration) from two molecules of ortho acid by heating. Pyrosulfuric acid is an example ( 2H2SO4 - H2O = H2S2O7). Ortho acid is the compound fully hydrated acid or its salts. Orthophosphoric acid is an example (2·H3PO4 = P2O5.3H2O), in contrast to the less hydrated form, pyrophosphoric acid (2·HPO3 = P2O5.H2O). Na2O5S2 is called sodium metabisulfite (2·HNaO3S - H2O). Sulfide is a compound having one or more sulfur atoms in which the sulfur is connected directly to a carbon, metal, or other nonoxygen atom; for example sodium sulfide, Na2S. Sulfide ion is S2- with oxidation number -2. Bisulfide ion is an anion formed by two sulfur atoms having an overall -2 charge, (S2)2-. Sulfamate is a salt of sulfamic acid (HSO3NH2). Calcium sulfamate Ca(SO3NH2)2 is an example.
APPEARANCE
85% or 88% or 90% min
INSOLUBLES IN WATER
Na2S2O3
Na2S2O5
NaHSO3
Na2CO3
HCOONa
Pb
0.0001% max
Fe
4.2 (Packing Group:II)
HAZARD OVERVIEW
GHS (Globally Harmonised System) Classification: Self-heating substances. Acute toxicity (Oral). Acute aquatic toxicity. Hazard statements: Self-heating: may catch fire. Harmful if swallowed. Toxic to aquatic life. Contact with acids liberates toxic gas
GHS
Danger
PICTOGRAMS


HAZARD STATEMENTS
H251-H302-H402
P STATEMENTS
P235 + P410
![]()
RISK PHRASES
7-22-31
SAFETY PHRASES
7/8-26-28-43