| STRONTIUM CHROMATE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 7789-06-2, 54322-60-0 |
|
| EINECS NO. | 232-142-6 | |
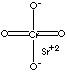
| FORMULA | SrCrO4 | |
| MOL WT. |
203.62 | |
| HS CODE | 284150 | |
|
TOXICITY |
Toxic | |
| SYNONYMS | C.I. pigment yellow 32; Strontium yellow; Deep lemon yellow; | |
| Strontium Dichromate, Chromic acid strontium salt (1:1); | ||
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | Yellow Powder | |
| MELTING POINT | ||
| BOILING POINT |
| |
| SPECIFIC GRAVITY | 3.89 | |
| SOLUBILITY IN WATER | soluble in hydrochloric acid, nitric acid and ammonia liquid | |
| pH | 5 - 8 | |
| VAPOR DENSITY | ||
| AUTOIGNITION |
| |
| NFPA RATINGS |
| |
REFRACTIVE INDEX |
| |
| FLASH POINT |
| |
| STABILITY | Stable under ordinary conditions | |
|
GENERAL DESCRIPTION & APPLICATIONS |
||
|
Chromium (symbol Cr and atomic
number 24) occurs
in the oxidation states 0, +2, +3, and +6 states. Element (0) and divalent chromium,
however, are unstable. Chromium (0) immediately produce
a thin oxide layer. Divalent chromium is easily oxidized to the
trivalent form in air. The trivalent and hexavalent oxidation
states are important in industry, which exit in their divalent anions called
chromate and dichromate respectively and an chromic anhydride
form called chromium trioxide (CrO3)
and chromic oxide (Cr2O3).
In industrial, chromium trioxide is called chromic acid.
The principal uses of chromium are in the metallurgical
processing of ferrochromium and other metallurgical products to impart corrosion resistance,
chiefly stainless steel. There are applications in chrome plating, anodizing
aluminium, and refractory processing of chrome brick. When combined with oxygen together
other metallic elements such as lead and potassium,
it forms various inorganic pigments. Chromium is used
in chemical processing to
produce chromic acid and chromates. Chromates are strong oxidants which will produce
many organic and inorganic
materials and used in the purification of chemicals. Chromates are used as rust and corrosion inhibitors
in diesel engines. Dichromate is converted to
chromic sulfate for tanning of leather. The reaction of chromium
with collagen raises the hydrothermal stability of the leather and renders it
resistant to bacterial attack. The reaction
with collagen is useful reaction in screen printing application and
in photography as a sensitizer for gelatin coatings. This Chromates and dichromates are used as pigments in paints
and in dyeing. Chrome colors include black, red, orange, green,
and yellow. Chromate salts contain the chromate ion, CrO4-2,
and have an intense yellow color. Dichromate salts contain the dichromate
ion, Cr2O7-2, and have an intense orange
color. Chromates are used as
mordant in dyeing cloth.
Chromic acid ( chromium trioxide, CrO3) is an odorless red deliquescent solid. Chromium trioxide is produced commercially by the reaction of sodium dichromate with concentrated sulfuric acid. It has been used mainly for chromium plating particularly in the production of automobiles and as a colorant in ceramics. Uses in other metal-finishing operations include aluminium anodizing, particularly on military aircraft; chemical conversion coatings, which provide both decoration and corrosion protection; and the production of phosphate films on galvanized iron or steel. It is a powerful oxidant and are utilized by controlled oxidations in organic synthesis. This compound is sensitive to moisture. Another significant oxygen compound, chromic oxide is prepared by calcining sodium dichromate with boric acid or by reducing sodium dichromate with carbon. Anhydrous chromic oxide is produced commercially from chromic hydroxide, dry ammonium dichromate, or sodium dichromate by heating with sulfur. Chromic oxide is a dark green, amorphous powder, forming hexagonal crystals on heating that are insoluble in water or acids. Most chromic oxide is used as a pigment. Anhydrous chromic oxide is known as the most stable green pigment used when heat, light and chemical resistance is required for glass, ceramics, and polymers. Its hydrate form is called Guignet's green and used as a green pigment, particularly for automotive finishes. Chromic compounds are also used in metallurgy in the manufacture of chromium metal and aluminium-chromium master alloys, in refractory brick, and as a chemical intermediate. They have good resistance to alkali and find application as colorant for latex paints. They are used in asphalt roofing and in camouflage paints. They are used as catalyst in the preparation of methanol, butadiene and high-density polyethylene. When used as a mild abrasive for polishing jewellery and fine metal parts, it is known as ��green rouge�� Chromic compounds are used extensively as pigments. Chromic acid finds applications in:
Strontium chromate is used as rust- and corrosion-resistant pigment in paints, varnishes and oil colors. It is used in water based wash primers, metal conditioners or in aluminium flake coatings, either alone or in combination with basic zinc chromate (solvent and vinyl based wash primer). Strontium chromate has also been used as an additive to control the sulfate content of solutions in electrochemical processes. |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
Yellow Powder | |
| STRONTIUM | 43% min | |
| CHROMIUM | 41% min | |
| CHLORIDE |
0.1% max | |
| SULPHATE |
0.2% max | |
|
NITRATE
|
0.05% max | |
| MOISTURE |
1.0% max | |
|
OIL ABSORPTION
|
25 ± 3 ( ml/100g ) | |
|
VOLATILE MATTE
|
0.5% max
| |
| COVERAGE |
150 max (g/m2) | |
|
PARTICLE SIZE |
97% max ( 325 mesh) | |
| TRANSPORTATION | ||
| PACKING | 25kgs, 50kgs in Bag | |
| HAZARD CLASS | 6.1 | |
| UN NO. | 2811 | |
| OTHER INFORMATION | ||
| Hazard Symbols: T, Risk Phrases: 45-22, Safety Phrases: 53-44 | ||