|
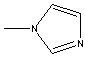
1-METHYLIMIDAZOLE |
||||||||||||||||||||||||||
|
PRODUCT IDENTIFICATION |
||||||||||||||||||||||||||
| CAS NO. |
616-47-7 |
|
||||||||||||||||||||||||
| EINECS NO. | 210-484-7 | |||||||||||||||||||||||||
| FORMULA | C4H6N2 | |||||||||||||||||||||||||
| MOL WT. | 82.12 | |||||||||||||||||||||||||
| H.S. CODE |
2933.29.9090 |
|||||||||||||||||||||||||
|
TOXICITY |
Mouse LD50 (Oral) 1400mg/kg | |||||||||||||||||||||||||
| SYNONYMS | N-Methylimidazole; 1-Methyl-1H-Imidazole; Methyl Imidazole; | |||||||||||||||||||||||||
|
Other RN: 69723-05-3; 110069-11-9; 120418-32-8; 142504-34-5; 674282-80-5; 864745-22-2; 955156-62-4 |
||||||||||||||||||||||||||
|
SMILES |
n1(ccnc1)C | |||||||||||||||||||||||||
|
CLASSIFICATION |
|
|||||||||||||||||||||||||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||||||||||||||||||||||||||
| PHYSICAL STATE | Clear to slightly yellow liquid | |||||||||||||||||||||||||
| MELTING POINT | -60 C | |||||||||||||||||||||||||
| BOILING POINT | 198 C | |||||||||||||||||||||||||
| SPECIFIC GRAVITY | 1.03 | |||||||||||||||||||||||||
| SOLUBILITY IN WATER |
miscible (1.00E+06 mg/l) |
|||||||||||||||||||||||||
| VAPOR DENSITY | 2.83 | |||||||||||||||||||||||||
| AUTOIGNITION |
524 C |
|||||||||||||||||||||||||
| pKa | 6.95 (at 25 C) | |||||||||||||||||||||||||
| log Pow | -0.08 (Octanol-water) | |||||||||||||||||||||||||
| VAPOR PRESSURE | 0.478 (mmHg) | |||||||||||||||||||||||||
| HENRY'S LAW | 8.01E-05 (atm-m3/mole at 25 C) | |||||||||||||||||||||||||
| OH RATE | 3.61E-11 (cm3/molecule-sec at 25 C Atmospheric) | |||||||||||||||||||||||||
| NFPA RATINGS | ||||||||||||||||||||||||||
|
REFRACTIVE INDEX |
1.4960 | |||||||||||||||||||||||||
| FLASH POINT |
92 C |
|||||||||||||||||||||||||
| STABILITY | Stable under ordinary conditions | |||||||||||||||||||||||||
|
GENERAL DESCRIPTION & EXTERNAL LINKS |
||||||||||||||||||||||||||
|
N-Methylimidazole
is used as an intermediate for pharmaceuticals, agrochemicals,
dyes, textile auxiliaries, pigments and other organic
chemicals. It is also used in polyurethane manufacturing
and epoxy resins as a curing agent. Wikipedia Linking:http://en.wikipedia.org/wiki/1-Methylimidazole http://www.rsc.org http://www.wag.caltech.edu/ http://www.sigmaaldrich.com |
||||||||||||||||||||||||||
| SALES SPECIFICATION | ||||||||||||||||||||||||||
|
APPEARANCE |
Clear to yellow liquid | |||||||||||||||||||||||||
|
ASSAY |
99.0% min |
|||||||||||||||||||||||||
|
WATER |
0.5% max |
|||||||||||||||||||||||||
| TRANSPORTATION | ||||||||||||||||||||||||||
| PACKING | 200kgs in drum | |||||||||||||||||||||||||
| HAZARD CLASS | 8 (Packing group:III) | |||||||||||||||||||||||||
| UN NO. | 3267 | |||||||||||||||||||||||||
| DESCRIPTION OF IMIDAZOLE | ||||||||||||||||||||||||||
|
Imidazole is a heterocyclic compound of five-membered diunsaturated ring
structure composed of three carbon atoms and two nitrogen atoms at nonadjacent
positions. The simplest member of the imidazole family is imidazole itself,
colorless to pale yellow crystalline solid with a weak aminelike odor; soluble
in water and alcohol, melts at 89 C, boils at 256 C. Imidazoles are poorly
soluble in water generally, but are dissolved in organic solvents, such as
chloroform, propylene glycol, and polyethoxylated castor oil. Imidazole ring is
found in histidine (an essential amino acid) and histamine, the decarboxylated
compound from histamine. Some imidazole compounds inhibit the biosynthesis of
ergosterol, required in cell membrane in fungal. They have antibacterial,
antifungal, antiprotozoal, and anthelmintic activity. Several distinct
phenylimidazoles are therapeutically useful antifungal agents against either
superficial or systemic infections. Thiabendazoles which have anthelmintic and
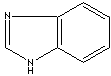
antifungal properties are imidazole class compounds. Benzimidazole is a dicyclic
compound having midazole ring fused to benzene. Benzimidazole structure is a
part of the nucleotide portion of vitamin B12 and the nucleus in some drugs such
as proton pump inhibitors and anthelmintic agents. Imidazole has two nitrogen
atoms.
The one is slightly acidic, while the other is basic. Imidazole and its
derivatives are widely used as intermediates in synthesis of organic target
compounds including pharmaceuticals, agrochemicals, dyes, photographic
chemicals, corrosion inhibitors, epoxy curing agents, adhesives and plastic
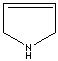
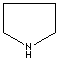
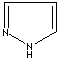
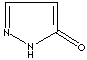
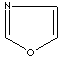
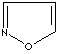
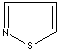
modifiers. Some imidazole analogues which contain nitrogen in five-membered ring
structure are:
|
||||||||||||||||||||||||||