|
ACETONE CYANOHYDRIN | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 75-86-5 |
|
| EINECS NO. | 200-909-4 | |
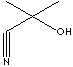
| FORMULA | (CH3)2C(OH)CN | |
| MOL WT. | 85.11 | |
| H.S. CODE | 2926.90 | |
|
TOXICITY |
Oral rat LD50: 18650 ug/kg | |
| SYNONYMS | 2-Hydroxy-2-methylpropanenitrile; 2-Methyllactonitrile; | |
| alpha-Hydroxyisobutyronitrile; 2-Hydroxy-2-methylpropionitrile; 2-Hydroxyisobutyronitrile; 2-methyl-Lactonitrile; 2-Cyano-2-propanol; 2-Hydroxyisobutyronitrile; 2-Methyllactonitrile; 2-Propanone, cyanohydrin; 2-Cyanopropan-2-ol; Acetoncianhidrinei; Acetoncianidrina; Acetoncyaanhydrine; Acetoncyanhydrin; Acetonkyanhydrin; Cyanhydrine d'acetone; | ||
| SMILES | condensation of acetone with hydrocyanic acid | |
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | Clear, colourless liquid | |
| MELTING POINT | -19 C | |
| BOILING POINT | 82 C | |
| SPECIFIC GRAVITY | 0.932 | |
| SOLUBILITY IN WATER | miscible | |
|
SOLVENT SOLUBILITY |
Soluble: alcohol, ether, acetone, benzene; Insoluble: petroleum ether | |
| AUTOIGNITION |
688 C | |
| pH |
3 (10% Sol.) | |
| VAPOR DENSITY | 2.93 | |
| NFPA RATINGS | Health: 4 Flammability: 2 Reactivity: 2 | |
| FLASH POINT |
73 C | |
| STABILITY | Stable under ordinary conditions. | |
|
APPLICATIONS |
||
| Cyanohydrins are organic compounds which contain both cyano and hydroxyl groups linked to the same carbon atom usually. Cyanohydrins are obtained from the reactions of aldehydes (or ketones) with hydrogen cyanide in base. They are versatile intermediates for the synthesis of organic compounds including amino nitriles; alpha-hydroxy acids, esters and amides; alpha-, beta- unsaturated acids, esters, nitriles as well as beta-aminoalcohols. Acetone Cyanohydrin is used to manufacture insecticides, pharmaceuticals and flavouring agents. It is an important intermedaite to prepare methacrylic acid and methacrylates. | ||
| SALES SPECIFICATION | ||
|
APPEARANCE |
yellowish liquid | |
| CONTENT |
93.0% min | |
| HYDROCYANIC ACID |
3.0% min | |
| TRANSPORTATION | ||
| PACKING | ||
| HAZARD CLASS | 6.1 (Packing group: I) | |
| UN NO. | 1541 | |
| OTHER INFORMATION | ||
| Hazard Symbols: T+, Risk Phrases: 26/27/28, Safety Phrases: 36/37/39-45 | ||
| GENERAL DESCRIPTION OF NITRILE | ||
| Nitrile is any of a family of organic compounds containing cyano group (-C��N) which is attached to a carbon atom and having the general formula RC��N. Their names are corresponding to carboxylic acids by changing '-ic acid' to '-onitrile', or '-nitrile', whichever preserves a single letter o. Examples are acetonitrile from acetic acid and benzonitrile from benzoic acid. Pendant nitriles are often named as ��cyano�� substituents. | ||