PRODUCT IDENTIFICATION
UN NO.
CLASSIFICATION
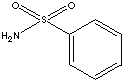
Sulfonamide
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
EXTERNAL LINKS & GENERAL DESCRIPTION
Wikipedia Linking (Sulfonamide)
Local: Amide is a group of organic chemicals with the general formula RCO-NH2 in which a carbon atom is attached to oxygen in solid bond and also attached to an hydroxyl group, where 'R' groups range from hydrogen to various linear and ring structures or a compound with a metal replacing hydrogen in ammonia such as sodium amide, NaNH2. Amides are divided into subclasses according to the number of substituents on nitrogen. The primary amide is formed from by replacement of the carboxylic hydroxyl group by the NH2, amino group. An example is acetamide (acetic acid + amide). Amide is obtained by reaction of an acid chloride, acid anhydride, or ester with an amine. Amides are named with adding '-ic acid' or '-oic acid' from the name of the parent carboxylic acid and replacing it with the suffix 'amide'. Amide can be formed from ammonia (NH3). The secondary and tertiary amides are the compounds which one or both hydrogens in primary amides are replaced by other groups. The names of secondary and tertiary amides are denoted by the replaced groups with the prefix capital N (meaning nitrogen) prior to the names of parent amides. Low molecular weight amides are soluble in water due to the formation of hydrogen bonds. primary amides have higher melting and boiling points than secondary and tertiary amides. Anilide is an amide derived from aniline by substitution of an acyl group for the hydrogen of NH2. Acetanilide is from acetic acid and aniline. Acetanilide is an odourless, white flake solid or crystalline powder (pure form); soluble in hot water alcohol, ether, chloroform, acetone, glycerol, and benzene;; melting point 114 C and boiling point 304 C; can undergo self-ignite at 545 C, but is otherwise stable under most conditions. Acetanilide which can be obtained by acetylation of aniline undergoes nitration at low temperature and yields highly the para-nitro products. Acetyl group can then be removed by acid-catalyzed hydrolysis to yield para-nitroaniline. Although the activating affection of the amino group can be reduced, the acetyl derivative remains an ortho/para-orientation and activating substituent. Examples of aromatic anilide are benzanilide, C6H5NHCOC6H5 or Carbanilide (N,N'-diphenylcarbamide). Some structural amides are;
- Acetamides
- Acrylamides
- Anilides
- Benzamides
- Naphthylamides
- Formamides
- Lactams
- Salicylamides
- Sulfonamides
- Thioamides
- Fatty amides
An amide is hydrolyzed to yield an amine and a carboxylic acid under strong acidic conditions. The reverse of this process resulting in the loss of water to link amino acids is wide in nature to form proteins, the principal constituents of the protoplasm of all cells. Acyl halides are the most reactive but amides the least reactive among carboxylic acid derivatives, as in order of "acyl halides > anhydrides > esters �� acids > amides". In homogeneous solvent systems, amides react with water only in the presence of strong acid or base catalysts under heating. Because of the nitrogen non-bonded electron pair with the carbonyl group, amides are very polar and the basicity is weaker than amines. Electrophiles bond to oxygen atom in preference to the nitrogen in an amide. One example of this reaction is the production of nitriles by dehydration of primary amides when treated with thionyl chloride. The addition of water to nitriles (carbon-nitrogen triple bond) gives an amide. Sulfonamides are analogs of amides in which the atom attached to oxygen in solid bond is sulfur rather than carbon. Sulfonamides react with alkyl halides, acid halides, sulfonyl halides, epihalohydrins, ketones and aldehydes under basic conditions. Benzamide, the simplest aromatic carboxylic amide, is used in the synthesis of various organic compounds. Polyamide is a polymer containing repeated amide groups such as various kinds of nylon and polyacrylamides.
Benzenesulfonamide is used as an intermediate for the synthesis dyes, photochemicals, and disinfectants. It is also used in electroplating and polyamide production industry.
APPEARANCE
1.0% max
50kgs in fiber drum, 9mts in container
PRICE INFORMATION