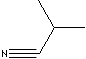
| ISOBUTYRONITRILE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO | 78-82-0 |
|
| EINECS NO. | 201-147-5 | |
| FORMULA | C3H7CN | |
| MOL WT. | 69.11 | |
| H.S. CODE | ||
|
TOXICITY |
||
| SYNONYMS | 2-Cyanopropane; 2-Methylpropionitrile; Isopropyl cyanide; | |
| 1-Cyano-1-methylethane; Dimethylacetonitrile; Isopropylkyanid; alpha-Methylpropanenitrile; 2-Methylpropanenitrile; Isobutironitrilo; Isobutyronitrile; | ||
| SMILES | ||
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | Clear liquid | |
| MELTING POINT | -72 C | |
| BOILING POINT | 107 C | |
| SPECIFIC GRAVITY | 0.76 | |
| SOLUBILITY IN WATER | ||
| AUTOIGNITION |
| |
| pH |
| |
| VAPOR DENSITY | 2.38 | |
| NFPA RATINGS | Health: 2 Flammability: 2 Reactivity: 0 | |
|
REFRACTIVE INDEX |
1.372 | |
| FLASH POINT |
8 C | |
| STABILITY | Stable under ordinary conditions. | |
|
GENERAL DESCRIPTION AND APPLICATIONS |
||
|
Nitrile is an organic compounds containing cyano group (-C��N, containing
trivalent nitrogen) which is attached to one carbon atom with the general
formula RC��N. Their names are corresponding to carboxylic acids by changing '-ic
acid' to the suffix, '-onitrile' which denotes only the ��N atom (triply bound)
excluding the carbon atom attached to it, or the suffix, '-carbonitrile' where
the carbon atom in the -CN is included, whichever preserves a single letter O.
Examples are acetonitrile from acetic acid and benzonitrile from benzoic acid.
The prefix, 'cyano-' is used as an alternative naming system to indicate the
presence of a nitrile group in a molecule for the compounds of salts and
organic derivatives of hydrogen cyanide (HC��N). Isocyanides are salts and hydrocarbyl
derivatives from the isomer, HN+��C-.
Sodium cyanide, NaCN; potassium
cyanide, KCN; calcium cyanide, Ca(CN)2; and hydrocyanic (or prussic) acid, HCN
are examples. Chemically, the simple inorganic cyanides resemble chlorides in
many ways. Organic nitriles act as solvents and are reacted further for various application including;
·
Extraction solvent for fatty acids,
oils and unsaturated hydrocarbons |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
Clear liquid | |
|
PURITY |
99.0% min | |
| DISTILLATION RANGE |
115 - 118 C | |
|
WATER |
0.2% max | |
| TRANSPORTATION | ||
| PACKING | 150kgs in drum | |
| HAZARD CLASS | 3 (Packing group: II) | |
| UN NO. | 2284 | |
| OTHER INFORMATION | ||
| Hazard Symbols: F T, Risk Phrases: 11-23/24/25-36/37/38, Safety Phrases: 36/37/39 | ||