OXALIC ACID DIHYDRATE
PRODUCT IDENTIFICATION
144-62-7 (Anhydrous), 6153-56-6 (Dihydrate)
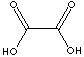
HOOCCOOH·2H2O
TOXICITY
SMILES
CLASSIFICATION
Dicarboxylic acid
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES (DIHYDRATE)
149 - 160 C (sublimes)
AUTOIGNITION
REFRACTIVE INDEX
NFPA RATINGS
163 C
EXTERNAL LINKS & GENERAL DESCRIPTION
http://www.all-about-food.org/
Oxalic acid is a chemical compound that has the simplest structure of all
dicarboxylic acids. The salt of this ubiquitous acid is called
oxalate (ethanedioate).
History of oxalic acid: Oxalic acid was first discovered in 1769 by the German pharmacist Johann
Christian Wiegleb in the plant Oxalis (Oxalis acetosella, from
which the name "oxalic acid" is derived). It was synthesized from inorganic
compounds by the chemist Friedrich Wöhler in 1924.
http://www.cyberlipid.org/
DICARBOXYLIC
ACIDS
Although the
dicarboxylic acids do not occur in appreciable amounts as components of animal
or vegetal lipids, they are in general important metabolic products of fatty
acids since they originate from them by oxidation. Dicarboxylic acids are
suitable substrates for preparation of organic acids for the pharmaceutical and
food industries. Furthermore, they are useful materials for the preparation of
fragrances, polyamides, adhesives, lubricants, and polyesters.
They have
the general type formula
HOOC-(CH2)n-COOH
In vegetal, a great variety of molecular forms of dicarboxylic
acids are found :
simple forms with a straight carbon chain or a branched chain
complex forms with a dicarboxylic acid and an alkyl side chain :
alkylitaconates
1 - Simple forms of dicarboxylic acids
Short-chain dicarboxylic acids are of great importance in the
general metabolism and up to n=3 they cannot be considered as lipids since their
water solubility is important. The simplest of these intermediates is oxalic
acid (n=0), the others are malonic (n=1), succinic (n=2) and glutaric (n=3)
acids.
Local: Oxalic Acid (also called Ethanedioic Acid) is a colourless, crystalline, toxic
organic compound belonging to the family of dicarboxylic acids; melting at 187
C;
soluble in water, alcohol, and ether. It occurs in the form of its metal salts
(usually calcium or potassium) in many plants. It is commercially manufactured
by heating sodium formate in the presence of an alkali catalyst to form sodium
oxalate, which should be converted to free oxalic acid when treated with
sulfuric acid. It is also prepared by oxidizing carbohydrates with nitric acid,
by heating saw dust with caustic alkalies or by fermentation of sugar solutions
in the presence of certain molds. Oxalic acid is the only possible compound in
which two carboxyl groups are joined directly; for this reason oxalic acid is
one of the strongest acids in organic compounds. Unlike other carboxylic acids,
oxalic acid (and formic acid) is readily oxidized and combine with calcium,
iron, sodium, magnesium, or potassium to form less soluble salts called
oxalates. Oxalic acid and oxalates are useful as reducing agents for
photography, bleaching, and rust removal. They are widely used as an purifying
agent in pharmaceutical industry, precipitating agent in rare-earth metal
processing, bleaching agent in textile and wood industry, rust-remover for metal
treatment, grinding agent, waste water treatment. acid rinse in laundries and
removing scale from automobile radiators.
Applications: Purifying agent,
Precipitating
agent, Bleaching
agent, Metal
treatment, Grinding
agent,
Waste water treatment,
Reducing agent
APPEARANCE
99.5% min
0.1% max
0.02% max
LOSS ON IGNITION
0.08% max
SULFATE
0.1% max
CHLORIDE
20ppm max
HEAVY METAL
10ppm max
GENERAL DESCRIPTION OF DICARBOXYLIC ACID
- Plasticizer for polymers
- Biodegradable solvents and lubricants
- Engineering plastics
- Epoxy curing agent
- Adhesive and powder coating
- Corrosion inhibitor
- Perfumery and pharmaceutical
- Electrolyte
There are almost infinite esters obtained from carboxylic acids. Esters are formed by removal of water from an acid and an alcohol. Carboxylic acid esters are used as in a variety of direct and indirect applications. Lower chain esters are used as flavouring base materials, plasticizers, solvent carriers and coupling agents. Higher chain compounds are used as components in metalworking fluids, surfactants, lubricants, detergents, oiling agents, emulsifiers, wetting agents textile treatments and emollients, They are also used as intermediates for the manufacture of a variety of target compounds. The almost infinite esters provide a wide range of viscosity, specific gravity, vapor pressure, boiling point, and other physical and chemical properties for the proper application selections.
|
C length (Straight) |
Product |
CAS # |
Melting Point |
Boiling Point |
|
C 2 |
Oxalic
Acid (Ethanedioic Acid) |
144-62-7 |
189 - 191 C |
Sublimes |
|
C 3 |
Malonic
Acid |
141-82-2 | 131 - 135 C |
Decomposes |
|
C 4 |
Succinic
Acid |
110-15-6 |
185 - 190 C |
235 C |
|
C 5 |
Glutaric
Acid |
110-94-1 |
95 - 99 C |
302 C |
|
C 6 |
Adipic
Acid |
124-04-9 |
151 - 153 C |
265 C at 100 mmHg |
|
C 7 |
Pimelic
Acid |
111-16-0 |
105 - 106 C |
212 C at 10 mmHg |
|
C 8 |
Suberic
Acid |
505-48-6 |
143 - 144 C |
230 C at 15 mmHg |
|
C 9 |
Azelaic
Acid |
123-99-9 |
100 - 103 C |
237 C at 15 mmHg |
|
C 10 |
Sebacic
Acid |
111-20-6 |
131 - 134 C |
294 at 100 mmHg |
|
C 11 |
Undecanedioic acid | 1852-04-6 |
109 - 110 C |
|
|
C 12 |
Dodecanedioic acid | 693-23-2 |
128 - 129 C |
245 C at 10 mmHg |
|
C 13 |
Brassylic acid (Tridecanedioic acid) |
505-52-2 |
112 - 114 C |
|
|
C 14 |
Tetradecanedioic acid | 821-38-5 |
126 - 128 C |
|
|
C 15 |
Pentadecanedioic acid | 1460-18-0 |
|
|
|
C 16 |
Thapsic acid (Hexadecanedioic acid) |
505-54-4 |
124 - 126 C |
|
|
C 18 |
Octadecanedioic acid |
871-70-5 |
|
|