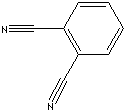
| PHTHALONITRILE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 91-15-6 |
|
| EINECS NO. | 202-044-8 | |
| FORMULA | C6H4(CN)2 | |
| MOL WT. | 128.13 | |
| H.S. CODE | ||
|
TOXICITY |
||
| SYNONYMS | o-Cyanobenzonitrile; o-Dicyanobenzene; | |
| 1,2-Benzenedicarbonitrile; o-Benzenedicarbonitrile; 1,2-Dicyanobenzene; o-Phthalodinitrile; o-PDN; Phthalic acid dinitrile; 1,2-Benzodinitrile; 1,2-Benzendikarbonitril; Ftalodinitril; Ftalonitril; o-Benzenedinitrile; | ||
|
SMILES |
||
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | almost white crystals with lumps | |
| MELTING POINT |
139 - 141 C |
|
| BOILING POINT | ||
| SPECIFIC GRAVITY | ||
| SOLUBILITY IN WATER | ||
| pH | ||
| VAPOR DENSITY | ||
|
AUTOIGNITION |
||
|
NFPA RATINGS |
Health: 2; Flammability: 0; Reactivity: 0 | |
|
REFRACTIVE INDEX |
||
| FLASH POINT | > 100 C | |
| STABILITY | Stable under ordinary conditions | |
|
GENERAL DESCRIPTION & APPLICATIONS |
||
| Phthalic Acid, also called Benzenedicarboxylic Acid with formula C6H4(COOH)2, is the name of any of three isomers. The ortho form (1,2-benzenecarboxylic acid) is called simply phthalic acid. It is a white crystals decomposing at 191°C and slightly soluble in water and ether. This compound is mainly produced and marketed in the form of its anhydride produced by the oxidation of orthoxylene and naphthalene. Its wide application is based on the ortho related carboxylic acid groups as their dehydration is highly reactive with broad processing conditions to produce various downstream products. It is used to make simple esters widely used as plasticizers. It is used as in making unsaturated polyester resins, alkyd resins, polyester polyols, dyes and pigments, halogenated anhydrides, polyetherimide resins, isatoic anhydride and insect repellents. The meta form is isophthalic acid (1,3-benzenecarboxylic acid). It is a white crystals subliming at 345°C slightly soluble in water, alcohol and acetic acid (insoluble in benzene). It is obtained by oxidizing meta-xylene with chromic acid, or by fusing potassium meta-sulphobenzoate, or meta-brombenzoate with potassium formate. IPA has excellent performance characteristics in coatings including excellent hardness, corrosion and stain resistance, hydrolytic stability of coatings and gel coats, excellent thermal stability and low resin color. It is a key ingredient in FRP markets for such products as marine, automotive, and corrosion resistant pipes and tanks. Polyesters containing isophthalic acid are also used extensively in industrial coatings applications for home appliances, automobiles, aluminum siding, and metal office furniture. It used as an intermediate for polyesters, polyurethane resins, plasticizers. The para form, known as terephthalic acid (1,4-benzenecarboxylic acid) is a combustible white powder insoluble in water, alcohol and ether; (soluble in alkalies), sublimes at 300°C. It can be produced by oxidizing caraway oil, a mixture of cymene and cuminol or by oxidizing para-diderivatives of benzene with chromic acid. TPA has been used mainly as a raw material of polyester fiber but lately it has been exploited for various uses such as non-fiber field, PET-bottle, PET-film and engineering plastics and as poultry feed additives. Phthalic acid derivatives are also widely used to make dyes, medicine, and synthetic perfumes, pesticides, and other chemical compounds. Phthalonitrile is used in organic synthesis ( such as phthalocyanine colorants, paint, polyamide fibre��catalyzers, phthalocyanine sulfonamide, flame retardants) and as an insecticide. | ||
| SALES SPECIFICATION | ||
|
APPEARANCE |
almost white crystals with lumps | |
| PURITY |
99.0% min |
|
| WATER |
0.1% max |
|
| TRANSPORTATION | ||
| PACKING | 25kgs in bag | |
| HAZARD CLASS | ||
| UN NO. | ||
| OTHER INFORMATION | ||
| European Hazard Symbols: T, Risk Phrases: 25, Safety Phrases: 36/37/39-45 | ||
| Nitrile is an organic compounds containing cyano group (-C��N, containing
trivalent nitrogen) which is attached to one carbon atom with the general
formula RC��N. Their names are corresponding to carboxylic acids by changing '-ic
acid' to the suffix, '-onitrile' which denotes only the ��N atom (triply bound)
excluding the carbon atom attached to it, or the suffix, '-carbonitrile' where
the carbon atom in the -CN is included, whichever preserves a single letter O.
Examples are acetonitrile from acetic acid and benzonitrile from benzoic acid.
The prefix, 'cyano-' is used as an alternative naming system to indicate the
presence of a nitrile group in a molecule for the compounds of salts and
organic derivatives of hydrogen cyanide (HC��N). Isocyanides are salts and hydrocarbyl
derivatives from the isomer, HN+��C-.
Sodium cyanide, NaCN; potassium
cyanide, KCN; calcium cyanide, Ca(CN)2; and hydrocyanic (or prussic) acid, HCN
are examples. Chemically, the simple inorganic cyanides resemble chlorides in
many ways. Organic nitriles act as solvents and are reacted further for various application including;
·
Extraction solvent for fatty acids,
oils and unsaturated hydrocarbons |
||