TRIETHANOLAMINE
PRODUCT IDENTIFICATION
TOXICITY
SMILES
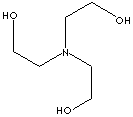
N(CCO)(CCO)CCO
CLASSIFICATION
Polyol, Amine, Aminoalcohol
EXTRA NOTES
EPA Pesticide Chemical Code 004208
PHYSICAL AND CHEMICAL PROPERTIES
360 C (Decomposes)
miscible with alcohol, methanol
10.5 ~ 11.5 at 149 g/l at 25 C
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
185 C
EXTERNAL LINKS & GENERAL DESCRIPTION
Drug Information Portal (U.S. National Library of Medicine) - Triethanolamine
PubChem Compound Summary - Triethanolamine
IPCS INCHEM - Triethanolamine
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Triethanolamine
http://www.ebi.ac.uk/ - Triethanolamine
http://www.ncbi.nlm.nih.gov/ - Triethanolamine
http://msdssearch.dow.com/Product Safety Assessment-DOW�� Triethanolamine
http://www.cosmeticsinfo.org/
Triethanolamine
is listed as Trialkylamines, trialkanolamines and their salts, and
Ethanolamine is listed as Monoalkylamines, monoalkanolamines and
their salts, in Annex III, Part I (substances which cosmetic products
must not contain except subject to the restrictions and conditions
laid down) of the Cosmetics Directive of the European Union. Triethanolamine
may be used in non-rinse-off and other cosmetics and personal care
products at a maximum concentration of 2.5%. Ethanolamine can be
used in cosmetics and personal care products if the secondary amine
is less than or equal to 0.5%. Neither Triethanolamine nor Ethanolamine
can be used with nitrosating systems, they must have purity of 99%
with a maximum secondary amine content of 0.5%, and a nitrosamine
content of 50 microg/kg or less, and the products must be in nitrite-free
containiers.
Local:
There are three ethanolamines called mono, di and tri-ethanolamine with
formula (CH2CH2OH)NH2,
(CH2CH2OH)2NH,
and (CH2CH2OH)3N
respectively. They are hygroscopic
viscous liquid or
semi-solid at room temperature. They are soluble in water, in alcohol and acetone, insoluble
in ether and benzene;
|
|
Monoethanolamine |
Diethanolamine |
Triethanolamine |
|
|
Formula |
(CH2CH2OH)NH2 |
(CH2CH2OH)2NH |
(CH2CH2OH)3N |
|
|
Specific Gravity |
1.018 |
1.0919 |
1.126 |
|
|
Freezing Point C |
10 - 11 |
28 |
21 - 22 |
|
|
Flash Point C |
91.0 |
166 |
210 |
|
|
Viscosity cP |
at 20 C |
24 |
crystalline |
crystalline |
|
|
at 30 C |
|
387 |
404 |
They are corrosive with a characteristic ammonia-like odor. their colors range from almost colorless to amber depending on purity. These substances decompose on heating and produce toxic and corrosive gases including nitrogen oxides. They are medium strongly basic and react with cellulose nitrate resulting in causing fire and explosion hazard. They react violently with strong acids and strong oxidants. Ethanolamines are produced from ethylene oxide reacted with ammonia. The principle product is monoethanolamine and secondary products of diethanolamine and triethanolamine are produced since ethylene oxide is reactive. They are the simplest members of the alkanolamine compounds. They have the physical and chemical characteristics of both alcohols and amines in one molecule. Ethanolamines stuctures are widely found in antihistamine drugs. In industrial field, monoethanolamine is an important raw material in the production of ethylenediamine. Ethanolamines are used as gas-scrubber in refinery and natural gas operations. They are widely used in the field of:
- Gas-scrubber
- Natural and refinery gas operations
- Hydrogen sulfide (H2S) and CO2 gas removal
- Textile Operation
- Softeners
- Lubricants
- Dye Leveling Agents
- Dispersants
- Durable Press
- Optical Brighteners
- Surfactants and Metalworking fluids
- Impart alkalinity
- Detergents
- Cosmetic formulations
- Acid neutralization
- Fatty acid soaps
- Emulsifiers
- Corrosion Inhibitors
- Others
- Concrete additives
- Cement admixtrue
- Urethane foams
- Agricultural products
- Photographic chemicals
- Biocides
- Oil well chemicals
- Rubber vulcanization accelerators
- Plasticizers
APPEARANCE
99.0% min
COLOR, APHA
50 max
MOISTURE
0.3% max
HAZARD OVERVIEW
Irritating to eyes. May cause skin and respiratory tract irritation. May cause an allergic skin reaction. Hygroscopic.
GHS
Warning
PICTOGRAMS

HAZARD STATEMENTS
H319: Causes serious eye irritation
PRECAUTIONARY STATEMENTS
P280: Wear protective gloves/protective clothing/eye protection/face protection
P305 + P351 + P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing
![]()
RISK PHRASES
36/37/38 Irritating to eyes, respiratory system and skin.
SAFETY PHRASES
26 In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
PRICE INFORMATION