|
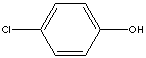
p-CHLOROPHENOL
| ||||||||||||||
|
PRODUCT IDENTIFICATION |
||||||||||||||
| CAS NO. | 106-48-9 |
| ||||||||||||
| EINECS NO. | 203-402-6 | |||||||||||||
| FORMULA | ClC6H4OH | |||||||||||||
| MOL WT. | 128.56 | |||||||||||||
| H.S. CODE | 2908.10 | |||||||||||||
|
TOXICITY |
Oral rat LD50: 670 mg/kg | |||||||||||||
| SYNONYMS |
4-Chlorophenolate;
P-Chlorophenic Acid; | |||||||||||||
| 4-Chlorophenol; 4-hydroxychlorobenzene; 4-Hydroxychlorobenzene; | ||||||||||||||
|
SMILES |
|
|||||||||||||
|
CLASSIFICATION |
|
|||||||||||||
| CHLORO-PHENOL COMPOUNDS | ||||||||||||||
| Chlorinated phenol compounds are toxic, colourless, weakly acidic, one or more of the chlorine atoms attached to the benzene ring of phenol. All chlorophenols are solids but only 2-chlorophenol is a liquid. The most general property toxicity is useful. They and compounds made from them are used as a bactericide and fungicide and preservative. 4-Chlorophenol a starting material for making germicides such as 2-Benzyl-4-chlorophenol; it can also be converted to an analgesic of acetophenetidin. 2,4-Dichlorophenol and formaldehyde form methylenebis compounds used as a mothproofing agent, an antiseptic, and a seed disinfectant; 2,4-dichlorophenol, with chloroacetic acid, forms 2,4-Dichlorophenoxyacetic acid (2,4-D), used as a selective weed-killer, systemic herbicide and defoliant, also used to increase the latex output of old rubber trees and in fruit drop control. trichlorophenols. 2,4,6-Trichlorophenol is used as a bactericide and fungicide. The 2,4,5-isomer has similar applications and also can be converted into Hexachlorophene or Thiobis(trichlorophenol) used as germicides in soap. and into Dimethyl trichlorophenyl phosphorothioate, a systemic agent effective against grubs in cattle; and into 2,4,5-Trichlorophenoxyacetic acid (2,4,5-T) or 2,4,5-Trichlorophenoxypropionic acid (2,4,5-TCPPA), both widely used as weed killers. Tetrachlorophenol is an insecticide and a bactericide and is used as a preservative for latex, wood, and leather. | ||||||||||||||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||||||||||||||
| PHYSICAL STATE | clear liquid | |||||||||||||
| MELTING POINT | 43 - 45 C | |||||||||||||
| BOILING POINT | 220 C | |||||||||||||
| SPECIFIC GRAVITY | 1.30 | |||||||||||||
| SOLUBILITY IN WATER | Soluble | |||||||||||||
|
SOLVENT SOLUBILITY |
||||||||||||||
| pH | Slightly acidic | |||||||||||||
| VAPOR DENSITY | ||||||||||||||
| AUTOIGNITION |
| |||||||||||||
| NFPA RATINGS | Health: 3; Flammability: 1; Reactivity: 0 | |||||||||||||
| REFRACTIVE INDEX |
||||||||||||||
| FLASH POINT | 98 C | |||||||||||||
| STABILITY | Stable under ordinary conditions | |||||||||||||
|
DESCRIPTION AND APPLICATIONS |
||||||||||||||
|
p-Chlorophenol is used as an intermediate for the synthesis of insecticides, herbicides, preservatives, antiseptics and disinfectants. It is also used in making medicines, dyes, aroma compounds and other organic chemicals. It is used as a solvent for extracting sulfur and nitrogen compounds from coal. When substituted benzene molecules undergo electrophilic substitution reactions, substituents on a benzene ring can influence the reactivity. Activating substituents that activate the benzene ring toward electrophilic attack can alter the reaction rate or products by electronically or sterically affecting the interaction of the two reactants. deactivating substituents removes electron density from the benzene ring, making electrophilic aromatic substitution reactions slower and more difficult than benzene itself. For example, a hydroxy or methoxy substituent in phenol and anisole increases the rate of electrophilic substitution, while a nitro substituent decreases the ring's reactivity. Electron donating substituents activate the benzene ring toward electrophilic attack, and electron withdrawing substituents deactivate the ring, making it less reactive to electrophilic attack. The strongest activating substituents are the amino (-NH2) and hydroxyl (-OH) groups.
Activating substituents generally direct substitution to the ortho and para positions where substitutions must take place. With some exceptions, deactivating substituents direct to the meta position. Deactivating substituents which orient ortho and para- positions are the halogens (-F, -Cl, -Br, -I) and -CH2Cl, and -CH=CHNO2 When disubstituted benzene molecules undergo electrophilic substitution reactions, a new substituent is directed depends on the orientation of the existing substituents and their individual effects; whether the groups have cooperative or antagonistic directing effects. Ortho position is the most reactive towards electrophile due to the highest electron density ortho positions. But this increased reactivity is countervailed by steric hindrance between substituent and electrophile. A nucleophilic substitution is a substitution reaction which the nucleophile displaces a good leaving group, such as a halide on an aromatic ring. This mechanism is called SNAr ( the two-step addition-elimination mechanism), where electron withdrawing substituents activate the ring towards nucleophilic attack. Addition-elimination reactions usually occur at sp2 or sp hybridized carbon atoms, in contrast to SN1 and SN2 at sp3. Chloro and bromobenzene reacts with the very strong base sodium amide (NaNH2) to give good yields of aniline. Other nucleophilic aromatic substitution mechanisms include benzyne mechanism and free radical (SRN1) mechanism. Common reactions of substituent groups on benzene ring include:
|
||||||||||||||
| SALES SPECIFICATION | ||||||||||||||
|
APPEARANCE |
clear liquid | |||||||||||||
|
ASSAY |
99.0% min | |||||||||||||
| MELTING POINT | 42 - 45 C | |||||||||||||
|
WATER |
0.5% max | |||||||||||||
| TRANSPORTATION | ||||||||||||||
| PACKING | 250kgs in drum | |||||||||||||
| HAZARD CLASS | 6.1 (Packing group: III) | |||||||||||||
| UN NO. | 2020 | |||||||||||||
|
OTHER INFORMATION |
||||||||||||||
| Hazard Symbols: XN C, Risk Phrases: 20/21/22/34,Safety Phrases: 2/28A | ||||||||||||||