|
2-ETHOXYETHYLACETATE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
PRODUCT IDENTIFICATION |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS NO. |
111-15-9 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EINECS NO. | 203-839-2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
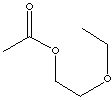
| FORMULA | CH3CO2CH2CH2OC2H5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MOL WT. | 132.16 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
H.S. CODE |
2915.35 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TOXICITY | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SYNONYMS | Cellosolve acetate; Ethylglycol acetate; 2EEA; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ethylene glycol monoethyl ether acetate; Ethyl Cellosolve Acetate; 2-Ethoxyethanol acetate; Glycol, monoethyl ether acetate; Ethylene glycol monethyl ether acetate; Ethoxyethyl acetate; Ethoxyethanol acetate; ethylene glycol ethyl ether acetate; 2-Aethoxy-aethylacetat (German); 2-Ethoxy-ethylacetaat (Dutch); 2-Ethoxyethyle, Acetate De (French); Acetato Di Cellosolve (Italian) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DESCRIPTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
CLASSIFICATION |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PHYSICAL STATE |
clear liquid, ether like odor |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MELTING POINT |
-60 C |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BOILING POINT |
156 C |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SPECIFIC GRAVITY | 0.975 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SOLUBILITY IN WATER | Soluble | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| pH |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VAPOR DENSITY |
4.72 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AUTOIGNITION |
310 C |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NFPA RATINGS | Health: 1; Flammability: 2; Reactivity: 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
REFRACTIVE INDEX |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FLASH POINT |
100 C |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| STABILITY | Stable under ordinary conditions | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SALES SPECIFICATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| APPEARANCE |
clear liquid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PURITY |
99.7% min (ASTM E611) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| WATER | 0.05% max ( ASTM D1364) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SPECIFIC GRAVITY | 0.975 (ASTM D891) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| COLOR, APHA | 15 max | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DISTILATION RANGE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IBP | 156 C (ASTM D1078) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DP | 160 C (ASTM D1078) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
APPLICATIONS |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Acetate is the
ester that an organic group replaces a hydrogen atom in -OH group of acetic acid
through reaction (typically condensation) with alcohols. Condensation is the
reaction in which two molecules having -OH groups are joined with eliminating a
water molecule from their -OH groups. They are produced by esterification reaction from acetic acid and the corresponding alcohol in the presence of strong acids like sulfuric acid. This reaction is reversible and acetate
can be hydrolyzed back into
alcohol and acetic acid in the presence of strong bases or strong acid, especially at elevated temperature.
The term acetate is also for the salt that
one or more of the hydrogen atoms of acetic acid are replaced by one or more
cations of the base, resulting in a compound containing the negative organic ion
of CH3COO-. Lower
acetate is a non-polar to weak polar aprotic solvent which have some solubility
portion in water. Its miscibility with water gets higher at elevated temperature.
Higher acetates have a
low
solubility in water and used as extraction solvents for fine chemicals
particularly for certain antibiotics. Organic acetates are
good solvents for a broad range of resins as they are miscible with almost all
common organic liquids. Due to their powerful solvency, high volatility and mild
odor, acetates are widely used as solvents for paints, coatings, adhesives, cellulose, plastics, fats,
wood stains. Additionally ether acetates series are also widely used as solvents. This surfactant-like structure provides the compatibility between water and a
number of organic solvents, and the ability to couple unlike phases. The main
products include ethyleneglycol
monoethyl ether acetate, ethyleneglycol monobutyl ether acetate,
and propyleneglycol monomethyl ether acetate.
Aromatic acetates
such as benzyl acetate are also useful solvent. Benzyl acetate has jasmine
like odor. Isoamyl acetate has a similar smell to both banana and pear. Acetates have characteristic fruity odor. They are used as component of perfumes
and flavorings.
They are used as chemical intermediate to manufacture pharmaceuticals, synthetic
flavorings, cleaners, and other organic compounds.
Cellosolves are monoether derivatives of ethylene glycol. They are excellent solvents, having solvent properties of both ethers and alcohols. Cellosolve and analogues are used as solvent for nitrocellulose, oils and resins and for many different purposes. They are used in automobile lacquers to retard evaporation and impart high gloss. They are used in printing inks, photographic film, lacquers, and nail polish. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TRANSPORTATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PACKING | 200kgs in drum | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HAZARD CLASS | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UN NO. |
1172 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OTHER INFORMATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Hazard
Symbols: T, Risk Phrases: 10-20/21/22-60-61, Safety Phrases:
53-9-16-33-45
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||