|
3-METHOXY-1-BUTANOL |
||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. |
2517-43-3 |
|
| EINECS NO. | 219-741-8 | |
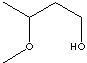
| FORMULA | CH3CH(OCH3)CH2CH2OH | |
| MOL WT. | 104.15 | |
| H.S. CODE |
2905.14 |
|
|
TOXICITY |
Oral rat LD50: 4000 mg/kg | |
| SYNONYMS |
Methoxybutanol; Butolether; 3-Methoxybutanol; |
|
| 3-Methoxy-n-butanol; 1,3-Butylene glycol monomethyl ether; 1,3-Butanediol 3-methyl ether; | ||
|
SMILES |
|
|
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | clear liquid | |
| MELTING POINT | -85 C | |
| BOILING POINT | 161 C | |
| SPECIFIC GRAVITY | 0.922 | |
| SOLUBILITY IN WATER |
miscible |
|
| pH |
|
|
| VAPOR DENSITY | 3.59 | |
| AUTOIGNITION |
335 C |
|
| NFPA RATINGS | Health: 1; Flammability: 2; Reactivity: 0 | |
|
REFRACTIVE INDEX |
1.4160 | |
| FLASH POINT |
46 C |
|
| STABILITY | Stable under ordinary conditions | |
|
APPLICATIONS |
||
|
Alcohols are widely used as solvents, fuels and chemical raw materials. Generally, hydroxyl group compounds are polar, which trends to promote solubility in water. But the carbon chain resist to solubility in water. Short chain alcohols (methanol, ethanol, and propanol) in which the hydroxyl group predominates are miscible in water. Butanol is moderately soluble because of the balance between the two opposing solubility trends. Higher alcohols are practically insoluble in water because of the hydrocarbon chain's trend is stronger. Alcohols are "protic" solvents. Protic refers to a hydrogen atom attached to an electronegative atom, oxygen. Polar protic solvents are compounds that can be represented by the general formula ROH of which water (H2O), methanol (CH3OH) and acetic acid (CH3COOH) are examples. Water-soluble alcohols, low-molecular weight products, are solvents for the manufacture of coatings, dyes and inks, plastics, flavorings, personal-care products, pharmaceuticals, and cleaners. The higher alcohols, slightly soluble in water or insoluble, can provide the proper balance of target properties when solvent-based solvents are formulated for desired viscosity, flowing and leveling, and curing rate and can be used as coupling agents in waterborne coatings. Alcohols are very weak acids as they lose H+ in the hydroxyl group. Alcohols undergoes dehydration reaction which means the elimination of water molecule replaced by a pi bond between two adjacent carbon atoms to form alkenes under heating in the presence of strong acids like hydrocloric acid or phosphoric acid. Primary and secondary alcohols can be oxidized to aldehydes and ketones respectively. Carboxylic acids are obtained from oxidation of aldehydes. Oxidation in organic chemistry can be considered to be the loss of hydrogen or gain of oxygen and reduction to gain hydrogen or loss of oxygen. Tertiary alcohols do not react to give oxidation products as they have no H attached to the alcohol carbon. Alcohols undergoes important reactions called nucleophilic substitution in which an electron donor replaces a leaving group, generally conjugate bases of strong acids, as a covalent substitute of some atom. One of important reaction of alcohol is condensation. Ethers are formed by the condensation of two alcohols by heating with sulfuric acid; the reaction is one of dehydration. Almost infinite esters are formed through condensation reaction called esterification between carboxylic acid and alcohol, which produces water. Alcohols are important solvents and chemical raw materials. Alcohols are intermediates for the production of target compounds, such as pharmaceuticals, veterinary medicines, plasticizers, surfactants, lubricants, ore floatation agents, pesticides, hydraulic fluids, and detergents. Carbinol is a primary alcohol with general formula RCH2OH. In carbinol nomenclature system, the term of carbinol is methanol itself and other groups are considered to have replaced one of the methanol hydrogen atoms to describe larger alcohols as derivatives of carbinol. This nomenclature system is particularly useful when the groups attached to the methanol carbon are large, aromatic, and cyclo groups. Benzyl alcohol is called phenylcarbinol or benzenecarbinol while benzyl carbinol is phenylethyl alcohol. 1-Butanol, a four carbon straight chain alcohol, is a volatile, clear liquid with a strong alcoholic odor; boils at 118 C, miscible with water. It is a highly refractive compound which corrodes some plastics, and rubbers. It is miscible with many organic solvents, and incompatible with strong oxidizers. It is flammable strongly with a luminous flame. It is used as a direct solvent and as an intermediate in the manufacture of other organic chemicals (e.g. Butyl acrylate and methacrylate, glycol ethers, butyl acetate, direct solvent use, plasticizers, amino resins, miscellaneous, including butylamines). Butanol and its derivatives continue to benefit from the long-term growth of water-based coatings formulations of all kinds. Analysts estimate that nearly 70 percent of all exterior architectural paints and as much as 85 percent of interior paints are now water-based. For butanol, butyl acrylate and butyl acetate, become increasingly important. Uses include :
Methoxybutanol's application is similar with n-butanol as a low volatile solvent, but better dissolving is expected. Methoxybutanol is used with n-butyl acetate to improve dissolving power, drying time, and flow. |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
clear liquid | |
|
ASSAY |
99.0% min |
|
| BUTYL ACETATE |
0.5% max |
|
|
ACID VALUE |
0.05 (mg KOH/g) |
|
|
COLOR |
10 max (Pt/Co Scale) |
|
|
WATER |
0.2% max |
|
| TRANSPORTATION | ||
| PACKING | 180kgs in drum | |
| HAZARD CLASS | 3 (Packing group: III) | |
| UN NO. | 1987 | |
| OTHER INFORMATION | ||
|
|
||