PRODUCT IDENTIFICATION
108-94-1
TOXICITY
SMILES
CLASSIFICATION
EXTRA NOTES
EPA Pesticide Chemical Code 025902
PHYSICAL AND CHEMICAL PROPERTIES
155 - 156 C
AUTOIGNITION
430 C
REFRACTIVE INDEX
NFPA RATINGS
46 C
EXTERNAL LINKS & GENERAL DESCRIPTION
http://encyclopedia2.thefreedictionary.com/
It is a colorless liquid with a characteristic odor. It has a melting point
of 6.55°C, a boiling point of 80.74°C, and a density of 0.778 g/cm3
at 20°C. Insoluble in water, cyclohexane is miscible with ether, acetone, and
benzene. It has two possible conformations —the “boat” and “chair” forms; the
latter predominates at normal temperatures. Cyclohexane is a component of virtually all petroleums, but it is present
only in small amounts. It is therefore produced in industry mainly by the
catalytic hydrogenation of benzene. It is used as a raw material for the
production of cyclohexanol and cyclohexanone (by oxidation with oxygen),
nitrocyclohexane (by the action of 30-percent nitric acid or nitrogen dioxide),
and cyclohexanone oxime (by nitrosation using NOCl); all of these substances are
intermediates in the production of caprolactam and adipic acid by catalytic
oxidation. Both caprolactam and adipic acid are used in the production of
polyamides.
Local:
Cyclohexane is the six-membered alicyclic
hydrocarbon consisting of six carbon atoms linked to each other to form a ring,
with each carbon atom bearing two hydrogen atoms, C6H12. A cyclic compound is an
organic compound that contains one or more closed rings of carbon atoms. The
term alicyclic refers to cyclic compound that behaves chemically like aliphatic
compounds (open-chain), which means the exclusion of carbocyclic compounds of
aromatic rings with an array of π-electrons characteristic. Cyclohexane is a
colorless, highly flammable, mobile liquid with a pungent odor. It is insoluble
in water and soluble in alcohol, ether, and almost organic solvents. Cyclohexane
is a non-corrosive and quick volatilize liquid and sublimes between -5 to 5 C.
Cyclohexane can exist in a number of interconvertible three-dimensional
conformations, the two simplest being are the chair and boat conformation and
others include the half-chair and twist-chair conformation. Cyclohexane can
cause irritation to the eyes and mucous membranes in workers. Repeated and
prolonged contact with skin may cause dermatitis. The substance has not been
shown to cause the hematologic changes associated with exposure to benzene.
Cyclohexane occurs naturally in crude oils. Some cyclohexane is recovered from
petroleum streams by fractionation. The bulky commercial production of
cyclohexane is based on hydrogenation of benzene in closed system. Cyclohexane
consumption is linked almost entirely to nylon production. Nylon is further
processed into fibers for applications in carpeting, automobile tire cord,
clothing, and other growing industrial fields. Cyclohexane is used as a solvent,
oil extractant, paint and varnish remover, dry cleaning material, and in solid
fuels. It has been used as a insecticide itself. Cyclohexane is used as a
chemical intermediate to produce target molecules. Natural compounds of one to
five alicyclic rings with great variety and complexity are found particularly in
steroids and terpenes. Cyclohexane structure, six membered-ring, is one of the
major skeleton in nature. Cyclohexane derivatives can be used for the synthesis
of pharmaceuticals, dyes, herbicides, plant growth regulator, plasticizers,
rubber chemicals, cycloamines and other organic compounds.
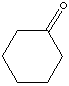
Cyclohexanone is a compound of ketone family. It is a clear oily liquid with an acetone like odor; slightly soluble in water but completely miscible with commom solvents. It is used as an industrial solvent and activator in oxidation reactions. It is used in the production of adipic acid, cyclohexanone resins, cyclohexanone oxime, caprolactam and nylon 6. Cyclohexanone having two alpha-carbons in the ring provides four alpha-hydrogens which can be substituted depending on the reaction conditions with remaining six hydrogens unreact.
APPEARANCE
99.5% min
20 max
WATER
0.05% max
PRICE INFORMATION