PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES (90% in Aq. Solution)
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
Stable under ordinary conditions
APPLICATIONS
APPEARANCE
ASSAY (G.C)
90.0% min
|
|
|
| 1-THIOGLYCEROL | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 96-27-5 |
|
| EINECS NO. | 202-495-0 | |
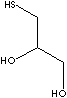
| FORMULA | HSCH2CH(OH)CH2OH | |
| MOL WT. | 108.16 | |
|
H.S. CODE |
||
|
TOXICITY |
|
|
| SYNONYMS | 3-Mercapto-1,2-propanediol; alpha-Thioglycerol; | |
| alpha-Thioglycerol; Glycerol-1-thiol; 1-Thio-2,3-propanediol; 2,3-Dihydroxypropanethiol; Monothioglycerin; 3-Mercaptopropane-1,2-diol; 3-Sulfanyl-1,2-propanediol; | ||
| DERIVATION | ||
|
CLASSIFICATION |
||
|
PHYSICAL AND CHEMICAL PROPERTIES (90% in Aq. Solution) |
||
| PHYSICAL STATE | clear to yellowish viscous solution | |
| MELTING POINT | ||
| BOILING POINT | 118 C at 5 mmHg | |
| SPECIFIC GRAVITY | 1.23 | |
| SOLUBILITY IN WATER | slightly soluble (miscible with alcohol, insoluble in ether) | |
| pH | ||
| VAPOR DENSITY |
| |
|
AUTOIGNITION |
| |
|
NFPA RATINGS |
||
|
REFRACTIVE INDEX |
||
| FLASH POINT |
| |
| STABILITY |
Stable under ordinary conditions | |
|
APPLICATIONS |
||
| The only existing three-carbon sugar alcohol (triitol) is glycerol. Low molecular weight thiols or their esters such as 2-mercaptoethanol, 1-thioglycerol and dithiothreitol denatures the proteins by reducing disulfide linkages leading to tautomerization and breaking up quaternary protein structure. They are involved in protein processing, induction of genes, oligonucleotide synthesis. They have been studied as anti-cancer agents by acting as alkylating agents to damage the cancer cell DNA. | ||
| SALES SPECIFICATION | ||
|
APPEARANCE |
clear to yellowish viscous solution | |
|
ASSAY (G.C) |
90.0% min |
|
| TRANSPORTATION | ||
| PACKING | | |
| HAZARD CLASS | ||
| UN NO. | ||
| OTHER INFORMATION | ||
| Hazard Symbols: XI, Risk Phrases: 36/37/38, Safety Phrases: 23 | ||
|
|
|