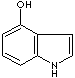
PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
97 - 99 C
REFRACTIVE INDEX
Health hazard: 2, Fire: 0, Reactivity Hazard: 0
Stable under ordinary conditions.
EXTERNAL LINKS & GENERAL DESCRIPTION
Drug Information Portal (U.S. National Library of Medicine) - 4-Hydroxyindole
PubChem Compound Summary - 4-Hydroxyindole
http://www.ebi.ac.uk/chebi/ - 4-Hydroxyindole
http://www.ncbi.nlm.nih.gov/ - 4-Benzyloxyindole
http://www.sigmaaldrich.com/
Application:·Reactant
for preparation of N-β-D-xylosyl-indole derivatives as SGLT2 inhibitors
for management of hyperglycemia in diabetes·Reactant for
preparation of small molecules targeting interaction between HIV-1
integrase and LEDGF/p75 cofactor·Reactant for preparation
of dopamine D2 receptor antagonists·Reactant for preparation
of SCD inhibitor MF-438·Reactant for preparation of carbon-11-labeled
4-aryl-4H-chromenes as new PET agents for imaging of apoptosis in
cancer·Reactant for preparation of indole/quinoline carbothioic
acid amide derivatives as HCV inhibitors
Local:
Indole,
benzopyrrole, is a yellow crystalline powder with unpleasant aroma. It has the
pyrrole ring (five-membered unsaturated ring structure composed of four carbon
atoms and one nitrogen atom) which is fused to benzene ring. There are
tautomerer called indolenine (unsubstituted 3H-indole) and structural isomer,
isoindole. But they are unstable. Indole occurs in some plants or in coal tar,
and is formed in the intestine during putrefaction and by certain cultures of
bacteria. it is commercially synthesized from phenylhydrazine and pyruvic acid.
Indole structure is a motif in nature. Prominent
examples include tryptophan (aromatic side chain amino acid), serotonin
(neurotransmitter), auxin (plant growth hormone), and indigo (plant colorant).
One more interesting point is all these compounds have functional branches at 3
position. Indole is used in perfumery and in preparing tryptophan,
one of the 20 amino acids commonly found in animal proteins. It has important
application in the industry of plant growth. It is used to prepare indoleacetic
acid (auxin) and other plant growth substances which help the development of
roots in plant. It is used to make selective herbicides. Indole and its
derivatives are widely used in making perfumes, dyes and agrochemicals as well
as in biologically
active ingredients and clinical diagnostics.
|
|
APPEARANCE
ASSAY
99.0% min
MELTING POINT
97 - 99 C
HAZARD OVERVIEW
OSHA Hazards: Irritant
GHS
PICTOGRAMS

HAZARD STATEMENTS
H315-H319-H335
P STATEMENTS
P261-P305 + P351 + P338
![]()
RISK PHRASES
36/37/38
SAFETY PHRASES
26-36