PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
REFRACTIVE INDEX
EXTERNAL LINKS & GENERAL DESCRIPTION
Drug Information Portal (U.S. National Library of Medicine) - 7-Azaindole
PubChem Compound Summary - 7-Azaindole
http://www.ebi.ac.uk/chebi/ - 7-Azaindole
http://www.ncbi.nlm.nih.gov/ - 7-Azaindole
http://pubs.rsc.org/
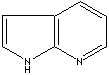
7-Azaindole
and its derivatives have been extensively investigated for uses
in biological probes and imaging. In contrast, there have been very
limited studies on the coordination chemistry and the applications
of 7-azaindole and derivatives in materials science and in chemical
bond activation. Our recent research has shown that 7-azaindolyl
and derivatives are excellent blue emitters for organic light emitting
diodes, they have rich coordination chemistry with both main group
elements and transition metal ions, and their metal complexes display
not only phosphorescence but also unusual and often unprecedented
reactivity toward C–H and C–X bonds. This critical review discusses
recent advances in these fields with focuses on new 7-azaindolyl
derivatives and their metal complexes developed by our group. The
luminescent properties and applications of 7-azaindolyl-based compounds
will be presented. The reactivity of Pt(II) complexes toward C–H
and C–X bonds, especially the steric impact of the bis(7-azaindolyl)
chelate ligands and bimetallic cooperativity will be discussed (86
references).
Local:
Indole, also called Benzopyrrole, is a yellow crystalline powder with unpleasant
aroma. It has the pyrrole ring (five-membered unsaturated ring structure
composed of four carbon atoms and one nitrogen atom) which is fused benzene
ring. There are tautomerer called indolenine (unsubstituted 3H-indole) and
structural isomer, isoindole. But they are unstable. Indole occurs in some
plants or in coal tar, and is formed in the intestine during putrefaction and by
certain cultures of bacteria. it is commercially synthesized from
phenylhydrazine and pyruvic acid. It is used in perfumery and in preparing
tryptophan, one of the 20 amino acids commonly found in animal proteins. It has
important application in the industry of plant growth. It is used to to prepare
indoleacetic acid (auxin) and other plant growth substances which help the
development of roots in plant. It is used to make selective herbicides. Indole
and its derivatives are widely
used in making perfumes, agrochemicals and medicines.
APPEARANCE
98.0% min
MOISTURE (K.F.)
0.5% max
LOSS ON DRYING
1.0% max
HAZARD OVERVIEW
OSHA Hazards: Irritant
GHS
PICTOGRAMS


HAZARD STATEMENTS
H302-H315-H318-H335
P STATEMENTS
P261-P280-P305 + P351 + P338
![]()
RISK PHRASES
22-37/38-41
SAFETY PHRASES
26-36/37/39