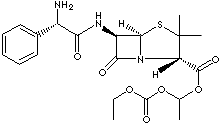
PRODUCT IDENTIFICATION
37661-08-8 (Hydrochloride)
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
SOLVENT SOLUBILITY
REFRACTIVE INDEX
Stable under ordinary conditions
APPLICATIONS
AMPICILLIN is a semisynthetic antibiotic, a member of the penicillin family of antibiotics. To extend the usefulness of the penicillins to the treatment of infections caused by gram-negative rods, the broad-spectrum penicillins (ampicillin, amoxicillin, carbenicillin, and ticarcillin) were developed. It is created by attaching acid radicals to the central structure of 6-aminopenicillanic acid. Chemically, it is D-(-)-6-(2-amino-2-phenylacetamido) -3,3-dimethyl-7 -oxo-4-thia-1-azabicyclo [3.2.0] heptane-2-carboxylic acid trihydrate. Similar in action to penicillin G--the most widely used natural penicillin--ampicillin (or alpha-aminobenzylpenicillin) is more stable in stomach acids and therefore may be given orally; it is also more active against certain strains of bacteria. It is used regularly to treat common urinary-tract infections, some respiratory infections, and bacterial meningitis in children, although many strains of Hemophilus influenzae (the most common cause of bacterial meningitis) are now resistant to ampicillin, and it commonly is used along with other drugs.The potential side effects of ampicillin are similar to those of other penicillins--i.e., chiefly allergic reactions ranging from skin rashes and hives to life-threatening anaphylactic shock (very rare). People who are allergic to other drugs in this family are also likely to react to ampicillin. The incidence of skin rashes is higher with ampicillin than with other penicillins, a factor that suggests a possible toxic reaction, as well as a truly allergic response. Bacampicillin is a modified ampicillin class semisynthetic penicillin for oral administration. It has the same actions and uses as ampicillin as it is hydrolyzed to ampicillin during absorption.
Members of ampicillin derivatives
|
Product |
CAS RN |
| Ampicillin sodium | 69-52-3 |
| Ampicillin | 69-53-4 |
| Penicillin G benzathine | 1538-09-6 |
| Metampicillin | 6489-97-0 |
| Ampicillin trihydrate | 7177-48-2 |
| Broadcillin | 8067-85-4 |
| Pivampicillin hydrochloride | 26309-95-5 |
| Epicillin | 26774-90-3 |
| Amoxicillin trihydrate | 26787-78-0 |
| Ampicillin benzathine | 33276-75-4 |
| Pivampicillin | 33817-20-8 |
| Amoxicillin sodium | 34642-77-8 |
| Bacampicillin hydrochloride | 37661-08-8 |
| Pivampicillin pamoate | 39030-72-3 |
| Talampicillin hydrochloride | 39878-70-1 |
| Pivampicillin probenate | 42190-91-0 |
| Talampicillin | 47747-56-8 |
| Bacampicillin | 50972-17-3 |
| Combipenix | 51004-51-4 |
| Ampicillinoic acid | 57457-66-6 |
| Amoxicillin anhydrous | 61336-70-7 |
| N-(N'-Methylasparaginyl)amoxicillin | 63329-59-9 |
| Aspoxicillin | 63358-49-6 |
| N-Propionylampicillin | 74226-27-0 |
| Amoxicillin-potassium clavulanate combination | 74469-00-4 |
| Amoxicillin-clavulanic acid mixture | 79198-29-1 |
| Lenampicillin hydrochloride | 80734-02-7 |
| Lenampicillin | 86273-18-9 |
| Sulacillin | 94935-63-4 |
APPEARANCE
ASSAY
WATER
1.0% max
Hazard Symbols: XN, Risk Phrases: 36/37/38-42/43, Safety Phrases: 22-26-36/37