PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
white to off-white crystalline powder
REFRACTIVE INDEX
NFPA RATINGS
AUTOIGNITION
FLASH POINT
GENERAL DESCRIPTION & APPLICATIONS
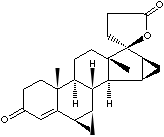
Spironolactone is a is a synthetic 17-lactone steroid belong to a class of drugs called potassium-sparing diuretics which inhibit the exchange of sodium for potassium and hydrogen ions in the distal tubule. It acts as a competitive aldosterone antagonist in the distal renal tubules. Anti-androgen effect is also utilized as a component of hormone replacement therapy for transgender, in the treatment of hair loss, female hirsutism and acne but it can lead to adverse effects. Spironolactone, as a potassium-sparing diuretic, increases the secretion of water and sodium, while decreasing the excretion of potassium. Aldosterone synthesis is stimulated by increased plasma angiotensin II or potassium levels, which are present in proportion to plasma sodium deficiencies. Spironolactone is mainly used in the treatment of refractory edema in patients with congestive heart failure, nephrotic syndrome, or hypokalemia. Spironolactone itself is a weak diuretic, and it is used in combination with other diuretics. Angiotensin II antagonist such as valsartan, eprosartan and telmisartan decrease the effects of aldosterone and used as antihypertensive. Drospirenone is a spironolactone analogue with antagonism of aldosterone. It is used in combination with an estrogen component as an oral contraceptive. Members of aldosterone antagonists include;
|
Aldosterone Antagonist |
CAS RN |
| 17alpha-Hydroxy-6beta,7beta-methylene-3-oxo-D-homo-17aalpha-pregna-4,16- dien-21- carboxylic acid | 81844-75-9 |
| 17beta-Hydroxy-12-alpha-etiojerv-4-en-3-one | 22785-18-8 |
| 17-Hydroxy-3-oxo-17 alpha-pregn-4-ene-7 alpha,21-dicarboxylic acid 7-methylester gamma-lactone | 41020-65-9 |
| 17-Hydroxy-3-oxo-17alpha-pregn-4-ene-7alpha,21-dicarboxylic acid, 7-(1-methylethyl) ester potassium | 41020-87-5 |
| 17-Hydroxy-3-oxo-19-nor-17alpha-pregn-4-ene-21-carboxylic acid gamma-lactone | 1722-54-9 |
| 18-Deoxyaldosterone | 2507-88-2 |
| 3,20-Dioxopregn-4-ene-18'-carboxaldehyde cyclic 18'-(1,2-ethandiylmercaptal) | 123376-04-5 |
| 5-Oxo-A-tetranor-17-alpha-pregna-9,11-diene-21-17-carbolactone | 76225-44-0 |
| 6,7-Epoxycanrenone | 114577-01-4 |
| 7alpha-(Methylthio)spironolactone S-oxide | 38753-75-2 |
| 7alpha-Thiospironolactone | 38753-76-3 |
| 7-Thiomethylspirolactone | 38753-77-4 |
| Aldatense | 76270-05-8 |
| Canrenoate Potassium | 2181-04-6 |
| Canrenone | 976-71-6 |
| Dicirenone | 41020-79-5 |
| Dihydroxy-6,7-dihydrocanrenone | 67483-35-6 |
| Drospirenone | 67392-87-4 |
| Eplerenone | 107724-20-9 |
| Mespirenone | 87952-98-5 |
| Mexrenoate potassium anhydrous | 41020-67-1 |
| Mexrenoate potassium | 43169-54-6 |
| Oxprenoate potassium | 76676-34-1 |
| Prorenoate | 49848-01-3 |
| Prorenoate potassium | 49847-97-4 |
| Prorenone | 49848-04-6 |
| Spironolactone | 52-01-7 |
| Spirorenone | 74220-07-8 |
APPEARANCE
white to off-white crystalline powder
pass Test A (IR)
ASSAY
99.0% min
LOSS ON DRYING
0.5% max
RESIDUE ON IGNITION
0.2% max
HEAVY METALS
10ppm max
MELTING POINT
160 - 166 C
OPTICAL ROTATION
-183° ~ -179°