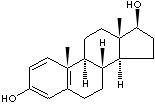
| ESTRADIOL | ||||||||||||||||||||||||||||||||||||||||
|
PRODUCT IDENTIFICATION |
||||||||||||||||||||||||||||||||||||||||
| CAS NO. | 50-28-2;
873662-39-6 (beta) 57-91-0 (alpha) |
|
||||||||||||||||||||||||||||||||||||||
| EINECS NO. | 200-023-8 | |||||||||||||||||||||||||||||||||||||||
| FORMULA | C18H24O2 | |||||||||||||||||||||||||||||||||||||||
| MOL WT. | 272.39 | |||||||||||||||||||||||||||||||||||||||
|
H.S. CODE |
2937.92.0000 |
|||||||||||||||||||||||||||||||||||||||
|
TOXICITY |
Rat LD (Subcutaneous):> 300mg/kg | |||||||||||||||||||||||||||||||||||||||
| SYNONYMS | 3,17-beta-Dihydroxyoestra-1,3,5-triene; 17-beta-OH-estradiol; | |||||||||||||||||||||||||||||||||||||||
| 1,3,5-Estratriene-3,17-beta-diol; 17-beta-Estra-1,3,5(10)-triene-3,17-diol; 17-beta-OH-oestradiol; 17-beta-Oestra-1,3,5(10)-triene-3,17-diol; Oestradiol; 17beta-Oestra-1,3,5(10)-triene-3,17-diol; 3,17-Epidihydroxyestratriene; 3,17-beta-Dihydroxy-1,3,5(10)-oestratriene; Estradiolum; 3,17-beta-Dihydroxyestra-1,3,5(10)-triene; 3,17-beta-Estradiol; Estradiol-17beta; 3,17-beta-Oestradiol; 3,17beta-Dihydroxyestra-1,3,5-triene; Dihydrofolliculin; Dihydrotheelin; Dihydroxyestrin; 3,17beta-Dihydroxyoestra-1,3,5-triene; Estra-1,3,5(10)-triene-3,17-beta-diol; Oestra-1,3,5(10)-triene-3,17-beta-diol; cis-estradiol; 3,17-epidihydroxyestratriene; Dihydrofollicular hormone; Estrovite; Femestral; Femogen; Gynergon; Gynestrel; Ovahormon; Ovocycline; Primofol; Progynon; Dimenformon; Dimenformon Prolongatum; Diogyn; 3,17-beta-Dihydroxy-1,3,5(10)-estratriene; | ||||||||||||||||||||||||||||||||||||||||
| SMILES |
C1[C@H]2[C@H]3[C@@H](c4c(cc(O)cc4)CC3)CC[C@@]2([C@H](O) C1)C |
|||||||||||||||||||||||||||||||||||||||
|
CLASSIFICATION |
Steroid, Steroid Hormone, Estrogen, Estrogen Modulator, Biochemicals ex Plants, Hormone Antagonist. |
|||||||||||||||||||||||||||||||||||||||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||||||||||||||||||||||||||||||||||||||||
| PHYSICAL STATE | white to off-white crystalline powder | |||||||||||||||||||||||||||||||||||||||
| MELTING POINT | 173 - 179 C | |||||||||||||||||||||||||||||||||||||||
| BOILING POINT |
|
|||||||||||||||||||||||||||||||||||||||
| SPECIFIC GRAVITY |
|
|||||||||||||||||||||||||||||||||||||||
| SOLUBILITY IN WATER |
practically insoluble (3.6 mg/l) |
|||||||||||||||||||||||||||||||||||||||
|
SOLVENT SOLUBILITY |
soluble in alcohol, acetone and dioxane, sparingly soluble in vegetable oils |
|||||||||||||||||||||||||||||||||||||||
| pH | ||||||||||||||||||||||||||||||||||||||||
| log Pow | 4.01 (Octanol-water) | |||||||||||||||||||||||||||||||||||||||
| VAPOR PRESSURE | 1.26E-08 (mmHg at 25 C) | |||||||||||||||||||||||||||||||||||||||
| HENRY'S LAW | 3.64E-11 (atm-m3/mole at 25 C) | |||||||||||||||||||||||||||||||||||||||
| OH RATE | 1.23E-10 (cm3/molecule-sec at 25 C Atmospheric) | |||||||||||||||||||||||||||||||||||||||
|
AUTOIGNITION |
|
|||||||||||||||||||||||||||||||||||||||
|
NFPA RATINGS |
Health: 2 Flammability: 0 Reactivity: 0 | |||||||||||||||||||||||||||||||||||||||
|
REFRACTIVE INDEX |
|
|||||||||||||||||||||||||||||||||||||||
| FLASH POINT |
|
|||||||||||||||||||||||||||||||||||||||
| STABILITY |
Stable under ordinary conditions |
|||||||||||||||||||||||||||||||||||||||
|
GENERAL DESCRIPTION & EXTERNAL LINKS |
||||||||||||||||||||||||||||||||||||||||
Estrogen is a mammalian estrogenic hormone naturally produced by follicle cells
of the vertebrate ovary, placenta, testis, and adrenal cortex. It is involved in
implantation of the fertilized ovum, acceleration of maturation, and the
maintenance of secondary sex characters and female reproductive organs such as
mammary glands, uterus, vagina, and fallopian tubes. Estradiol is most potent
mammalian estrogenic hormone. It is produced semi-synthetically for estrogen
replacement therapy in the treatment of female hypogonadism, vasomotor symptoms,
abnormal uterine bleeding, and primary ovarian failure. It is also used as a
feed additive as a growth promoter. There are two isomers: estradiol-17beta (the
principally active one) and estradiol-17alpha (inactive). It provokes ICSH
(interstitial-cell-stimulating hormone) secretion and proliferation of the human
endometrium. Estrogen includes
Wikipedia Linking: http://en.wikipedia.org/wiki/Estradiol http://biomarkers.uchicago.edu/
|
||||||||||||||||||||||||||||||||||||||||
| SALES SPECIFICATION | ||||||||||||||||||||||||||||||||||||||||
|
APPEARANCE |
white to off-white crystalline powder | |||||||||||||||||||||||||||||||||||||||
|
IDENTIFICATION |
Pass Test A(IR), B(UV) |
|||||||||||||||||||||||||||||||||||||||
|
ASSAY |
97.0
- 103.0%
|
|||||||||||||||||||||||||||||||||||||||
|
OPTICAL ROTATION |
+76° ~ +83° | |||||||||||||||||||||||||||||||||||||||
|
MELTING POINT |
173 - 179 C |
|||||||||||||||||||||||||||||||||||||||
|
WATER |
3.5% max |
|||||||||||||||||||||||||||||||||||||||
|
IMPURITY |
Individual impurity 0.2% max, Total impurity 0.5% max |
|||||||||||||||||||||||||||||||||||||||
| TRANSPORTATION | ||||||||||||||||||||||||||||||||||||||||
| PACKING |
|
|||||||||||||||||||||||||||||||||||||||
| HAZARD CLASS | ||||||||||||||||||||||||||||||||||||||||
| UN NO. | ||||||||||||||||||||||||||||||||||||||||
| OTHER INFORMATION | ||||||||||||||||||||||||||||||||||||||||
| Hazard Symbols: T, Risk Phrases: 45-63, Safety Phrases: 53-26-36/37/39 | ||||||||||||||||||||||||||||||||||||||||