GINGEROL
PRODUCT IDENTIFICATION
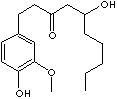
C17H26O4
294.39
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
Stable under ordinary conditions.
EXTERNAL LINKS & GENERAL DESCRIPTION
Wikipedia Linking: http://en.wikipedia.org/wiki/Gingerol
Objective: There is increasing interest in the study of the antioxidant actions of plant phenolic compounds as evidence shows that consumption of plant products rich in these compounds contributes to protection from a number of ailments including cardiovascular diseases. In the present study, the antioxidant effects of selected phenolic compounds from dietary sources, namely barbaloin, 6-gingerol and rhapontin, were investigated. Methods: Low-density lipoprotein (LDL), erythrocytes and erythrocyte membranes were subjected to several in vitro oxidative systems. The antioxidant effects of the phenolic compounds were assessed by their abilities in inhibiting hemolysis and lipid peroxidation of LDL and erythrocyte membranes, and in protecting ATPase activities and protein sulfhydryl groups of erythrocyte membranes. Results: 6-Gingerol and rhapontin were found to exhibit strong inhibition against lipid peroxidation in LDL induced by 2,2 -azobis(2-amidinopropane) hydrochloride (AAPH) and hemin while barbaloin possessed weaker effects. A similar order of antioxidant potencies among the three compounds was observed on the lipid peroxidation of erythrocyte membranes in a tert-butylhydroperoxide (tBHP)/hemin oxidation system. On the other hand, barbaloin and rhapontin were comparatively stronger antioxidants than 6-gingerol in preventing AAPH-induced hemolysis of erythrocytes. Among the three compounds, only barbaloin protected Ca2 -ATPase and protein sulfhydryl groups on erythrocyte membranes against oxidative attack by tBHP/hemin. Interestingly, rhapontin demonstrated protective actions on Na /K -ATPase in a sulfhydryl group-independent manner under the same experimental conditions. Conclusions: In view of their protective effects on LDL and erythrocytes against oxidative damage, these phenolic compounds might have potential applications in prooxidant state-related cardiovascular disorders. (http://www.jacn.org/)
The maturity of ginger rhizomes was studied by measuring their moisture content, fibre, density, and 6-gingerol content. Ginger rhizomes were harvested and divided into three groups according to their age of 4-6, 7-9, and 10-12 months. The results revealed that as ginger rhizomes age, moisture content and density decreased, while fibre and 6-gingerol content increased. Therefore, ginger maturity with the age of 10-12 months was used in the drying process. The effects of the drying aids maltodextrin and liquid glucose and inlet air temperatures on the properties of ginger powders were assessed. Moisture content, water activity, bulk density, water adsorption index, 6-gingerol content, and colour values of ginger powders decreased with increasing inlet air temperatures. Particle size, solubility, and water solubility index increased with increasing inlet air temperatures (p 6 0.05). Moisture content, water activity, water adsorption index, and particle size decreased with increasing the drying aids. Solubility, water solubility index, and yield increased with increasing the drying aids. The addition of 5% liquid glucose and an inlet air temperature of 120 °C resulted in good properties and the highest 6-gingerol content of the ginger powders. It was noted that liquid glucose could function as a drying aid and as an encapsulating agent. (http://www.scienceasia.org/)
Gingerol is the principal flavor and odor constituent of ginger, structurally similar to vanilloid. It provides the hot sensation like farnesol, gingerone, eudesmol, eucalyptol, shogaol, zingiberene, zingiberol and capsaicin. Various chain length gingerol exist such as 6-gingerol, 8-gingerol and 10-gingerol. During cooking, gingerol is converted to zingerone. Gingerol is used as a flavor and in perfumery to be responsible for spicy aroma.
APPEARANCE
CONTENT
5% (Ginger P.E)
PRICE
INFORMATION