PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
REFRACTIVE INDEX
NFPA RATINGS
AUTOIGNITION
FLASH POINT
GENERAL DESCRIPTION & APPLICATIONS
- Dipeptide: a molecule consisting of two amino acids
- Tripeptide a molecule consisting of three amino acids
- Octapeptide: a molecule consisting of eight amino acids
- Oligopeptide: a polypeptide composed of approximately 2-12 amino acids length (or 3 to 40 component amino acids)
- Polypeptide: a single molecule composed of approximately 13 or more amino acids.
- Protein: a linear polypeptides that are normally synthesized on ribosomes.
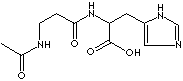
Carnosine is a dipeptide composed of ß-alanine and L-histidine. It is found in the brain, and in skeletal muscle and particularly in the olfactory pathways in which copper concentration is high. Carnosine may play a role as a neurotransmitter. It has a imidazole moiety that confers anti-oxidant activity. Carnosine performs the function of anti-glycation, pH buffering and metal chelation (particularly Cu2+). Carnosine compounds are used as nti-ulcer agent. Zinc L-carnosine (polaprezinc) stimulates bone growth and acts as an anti-ulcer agent.
Carnosine related molecules
|
Product |
CAS RN |
| Carnosine | 305-84-0 |
| Anserine | 584-85-0 |
| Homocarnosine | 3650-73-5 |
| L-Anserine nitrate | 10030-52-1 |
| Homocarnosine sulfate | 19841-48-6 |
| N-Acetylcarnosine | 56353-15-2 |
| Carcinine | 56897-53-1 |
| Polaprezinc | 107667-60-7 |
APPEARANCE
Conforms (IR)
PURITY
98.0% min
6.5% max
10ppm max