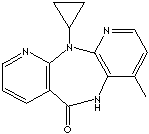
NEVIRAPINE
PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
white or off-white crystalline powder
REFRACTIVE INDEX
NFPA RATINGS
AUTOIGNITION
FLASH POINT
112 C
GENERAL DESCRIPTION & APPLICATIONS
Nevirapine is a non-nucleoside reverse transcriptase inhibitor of HIV-1 (human immunodeficiency virus-1). Reverse transcriptase is an enzyme that can transcribe RNA into DNA to copy genetic information from RNA to DNA, therefore also called RNA-directed DNA polymerase. Reverse transcriptase inhibitor is an antiretroviral agent that blocks activity of the reverse transcriptase of a retrovirus. Every retrovirus has a reverse transcriptase enzyme which transcribes the genetic information from its RNA into DNA. Protease inhibitors are the second class of antiretroviral drugs for the treatment of HIV.
Aantiretroviral agents
- Non-nucleoside reverse transcriptase inhibitors
- Delavirdine [CAS #: 136817-59-9]
- Delavirdine mesylate [CAS #: 147221-93-0]
- Efavirenz [CAS #: 154598-52-4]
- Nevirapine [CAS #: 129618-40-2]
- Nucleoside/Nucleotide reverse transcriptase inhibitors
- Abacavir [CAS #: 136470-78-5]
- Abacavir succinate [CAS #: 168146-84-7]
- Abacavir sulfate [CAS #: 188062-50-2]
- Adefovir [CAS #: 106941-25-7]
- Adefovir dipivoxil [CAS #: 142340-99-6]
- Combivir (Lamivudine/Zidovudine mixture) [CAS #: 165456-81-5]
- Didanosine [CAS #: 69655-05-6]
- Emtricitabine [CAS #: 143491-57-0]
- Lamivudine [CAS #: 134678-17-4]
- Lamivudine sulfoxide [CAS #: 160552-55-6]
- Pradefovir mesylate [CAS #: 625095-61-6]
- Stampidine [CAS #: 217178-62-6]
- Stavudine [CAS #: 3056-17-5]
- Tenofovir [CAS #: 147127-20-6]
- Tenofovir disoproxil [CAS #: 201341-05-1]
- Tenofovir Disoproxil Fumarate [CAS #: 202138-50-9]
- Trizivir (Abacavir sulfate/Lamivudine/Zidovudine mixture) [CAS #: 364057-50-1]
- Zalcitabine [CAS #: 7481-89-2]
- Zidovudine [CAS #: 30516-87-1]
- Zidovudine sodium salt [CAS #: 106060-86-0]
- Protease inhibitors
- Amprenavir [CAS #: 161814-49-9]
- Atazanavir [CAS #: 198904-31-3]
- Atazanavir sulfate [CAS #: 229975-97-7]
- Fosamprenavir [CAS #: 226700-79-4]
- Fosamprenavir calcium [CAS #: 226700-81-8]
- Fosamprenavir sodium [CAS #: 226700-80-7]
- Indinavir [CAS #: 150378-17-9]
- Indinavir hydrate [CAS #: 180683-37-8]
- Indinavir Sulfate [CAS #: 157810-81-6]
- Kaletra (Lopinavir/Ritonavir mixture) [CAS #: 369372-47-4]
- Lopinavir [CAS #: 192725-17-0
- Nelfinavir [CAS #: 159989-64-7]
- Nelfinavir mesylate [CAS #: 159989-65-8]
- Ritonavir [CAS #: 155213-67-5]
- Saquinavir [CAS #: 127779-20-8]
- Saquinavir mesylate [CAS #: 149845-06-7]
- Tipranavir [CAS #: 174484-41-4]
- Tipranavir disodium [CAS #: 191150-83-1]
APPEARANCE
white or off-white crystalline powder
pass Test A (IR), B (HPLC)
ASSAY
98.0 - 102.0%
LOSS ON DRYING
0.5% max
RESIDUE ON IGNITION
0.1% max
HEAVY METALS
10ppm max
SOLVENT RESIDUE
0.2% max (EtOH)