PRODUCT IDENTIFICATION
129-06-6 (Sodium)
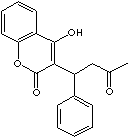
H.S. CODE
TOXICITY
4-Hydroxy-3-(3-oxo-1-phenyl-butyl)-cumarin;
(Phenyl-1 acetyl-2 ethyl) 3-hydroxy-4 coumarine; 1-(4'-Hydroxy-3'-coumarinyl)-1-phenyl-3-butanone; Coumarin 2000; 3-(1'-Phenyl-2'-acetylethyl)-4-hydroxycoumarin; Warfarin; Warfarina; Warfarine; Warfarine; Warfarinum; 3-(alpha-Phenyl-beta-acetylaethyl)-4-hydroxycumarin; 3-(alpha-Phenyl-beta-acetylethyl)-4-hydroxycoumarin; 4-Hydroxy-3-(3-oxo-1-fenyl-butyl)cumarine; 4-Hydroxy-3-(3-oxo-1-phenylbutyl)-2H-1-benzopyran-2-one; 4-Hydroxy-3-(3-oxo-1-phenylbutyl)coumarin; 4-Idrossi-3-(3-oxo-1-fenil-butil)-cumarine; Brumolin; Coumadin; Coumafen; Coumafene; Coumaphene; DL-3-(alpha-Acetonylbenzyl)-4-hydroxycoumarin; Dethmor; Dethnel; Kumader; Maveran; Ratoxin; Ratron; Rodafarin; Zoocoumarin; Zoocoumarin; Other RN: 5543-56-6, 56573-89-8. Other RN (Sodium salt): 12795-55-0, 51821-81-9, 859043-62-2
CLASSIFICATION
EXTRA NOTES
EPA
Pesticide Chemical Code 086002
An anticoagulant that acts by inhibiting the synthesis of vitamin K-dependent
coagulation factors. Warfarin is indicated for the prophylaxis and/or treatment
of venous thrombosis and its extension, pulmonary embolism, and atrial
fibrillation with embolization. It is also used as an adjunct in the prophylaxis
of systemic embolism after myocardial infarction. Warfarin is also used as a
rodenticide.
Warfarin is a trademark of Wisconsin Alumni Research Foundation
PHYSICAL AND CHEMICAL PROPERTIES
REFRACTIVE INDEX
Health hazard: 1, Fire: 0, Reactivity Hazard: 0
EXTERNAL LINKS & GENERAL DESCRIPTION
Drug Information Portal (U.S. National Library of Medicine) - Warfarin
PubChem Compound Summary - Warfarin
IPCS INCHEM - Warfarin
Drug Bank - Warfarin
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Warfarin
http://www.ebi.ac.uk/chebi/ - Warfarin
http://www.ncbi.nlm.nih.gov/ - Warfarin
Human Metabolome Database - Warfarin
http://pmep.cce.cornell.edu/
INTRODUCTION:
Warfarin was the first anticoagulant rodenticide introduced and
was first registered for use in the United States in 1952 (4, 13).
Warfarin is used for controlling rats and house mice in and around
homes, animal and agricultural premises, and commercial and industrial
sites. It is odorless and tasteless and effective in very low dosages.
Action is not rapid; usually about a week is required before a marked
reduction in the rodent population is noticeable. Rodents do not
tend to become bait-shy after once tasting warfarin; they continue
to consume it until its anti-clotting properties have produced death
through internal hemorrhaging. The prothrombin content of the blood
is reduced and internal bleeding is induced. Repeated ingestion
is needed to produce toxic symptoms. This rodenticide can be used
year-after-year wherever a rodent problem exists. Mice are harder
to control than rats, and complete control may take a longer period.
Recently, resistant strains of rats and mice are developing. Warfarin
comes in water soluble, ready-to-use bait, concentrate, powder,
liquid concentrate, nylon pouch, coated talc and dust formulations.
The compound also comes in mixed formulations with pindone, calciferol,
and sulphaquinoxaline. It is considered compatible with other rodenticides).
Warfarin is only slightly dangerous to humans and domestic animals
when used as directed, but care must be taken with young pigs, which
are especially susceptible
http://www.bbc.co.uk/
Why
are anticoagulants needed: Blood clotting, the mechanism by which
the blood sticks together to form small solid clots is a natural
and vital function of the body. The blood has a complex system which
regulates when or how clots form. More than 30 substances in the
blood are known to affect clotting and it's essential that the balance
of these clotting factors is right. Blood coagulation is triggered
by blood cells called platelets which, through a series of chemical
reactions, produce a substance called thrombin. This converts a
blood protein fibrinogen to fibrin which then create a series of
tiny threads which lead the plasma in the blood to become sticky.
The process protects the body from excessive bleeding, ensuring
that a clot forms at the site of a wound or injury - either inside
or outside the body. There is a constant state of formation and
breakdown of tiny clots throughout the body. In some conditions
some of the clotting chemicals are deficient or not working as they
should, and the blood doesn’t form clots properly and there is
a risk of haemorrhage. Alternatively variation in the levels of
clotting factors can make the blood form clots too easily and there
is a risk of clots forming where or when they are not wanted, leading
to life-threatening conditions such as strokes and heart attacks.
Other conditions such as abnormal heart rhythms or operations can
trigger normal blood to clot leading to complications. In these
cases anticoagulant drugs can help to prevent clot formation.
Local:
Warfarin is a synthetic coumarin anticoagulant which inhibits the hepatic
synthesis of the vitamin K-dependent coagulation factors. Its chemical
designation is 3-(alpha-acetonylbenzyl) -4 -hydroxycoumarin; racemic mixture of
the R and S enantiomers. It is also used as a rodenticide due to the property
of causing fatal hemorrhaging. Its salts (sodium or potassium) of warfarin are administered
orally, intravenously, or intramuscularly. Warfarin sodium is a white, odorless,
crystalline powder; soluble in water, in alcohol; slightly soluble in chloroform
and in ether.
|
|
APPEARANCE
98.0% min
LOSS ON DRYING
1.0% max
HAZARD OVERVIEW
OSHA Hazards:Target Organ Effect, Highly toxic by ingestion, Harmful by skin absorption., Teratogen. Target Organs:Blood
GHS
PICTOGRAMS


HAZARD STATEMENTS
H300
Fatal if swallowed.
H312 Harmful in contact with skin.
H360
May damage fertility or the unborn child.
H372 Causes damage
to organs through prolonged or repeated exposure if swallowed.
H402
Harmful to aquatic life.
PRECAUTIONARY STATEMENTS
P201
Obtain special instructions before use.
P264 Wash hands thoroughly
after handling.
P280 Wear protective gloves/ protective clothing.
P301
+ P310 IF SWALLOWED: Immediately call a POISON CENTER or doctor/
physician.
P308 + P313 IF exposed or concerned: Get medical advice/
attention
![]() T
Toxic
T
Toxic
RISK PHRASES
6 Explosive
with or without contact with air
48/25 Toxic: danger of
serious damage to health by prolonged exposure if swallowed
52/53 Harmful
to aquatic organisms, may cause long-term adverse effects in the
aquatic environment
SAFETY PHRASES
45 In
case of accident or if you feel unwell, seek medical advice immediately
(show label where possible)
53 Avoid exposure - obtain special
instruction before use
61 Avoid release to the environment.
Refer to special instructions safety data sheet
PRICE INFORMATION