PRODUCT IDENTIFICATION
76738-62-0
H.S. CODE
TOXICITY
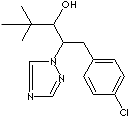
[(±)-(R',R')-beta-[(4-Chlorophenyl)methyl]-alpha-(1,1-dimethylethyl)-1H-1,2,4-triazole-1- ethanol; (RS,3RS)-1-(4-Chlorophenyl)-4,4-dimethyl-2-(1H-1,2,4-triazol-1-yl)pentan-3-ol; (2RS,3RS)-1-(4-Chlorophenyl)-4,4-dimethyl-2-(1H-1,2,4-triazol-1-yl)pentan-3-ol; Parlay; Trimmit; beta-((4-Chlorophenyl)methyl)-alpha-(1,1-dimethylethyl)-1H- 1,2,4-triazole-1-ethanol; Other RN: 66346-04-1, 77108-06-6
n1c[n@]([C@@H]([C@H](C(C)(C)C)O)Cc2ccc(cc2)Cl)nc1.n1c[n@](nc1) [C@H]([C@@H](C(C)(C)C)O)Cc1ccc(cc1)Cl
CLASSIFICATION
Plant Growth regulator, Herbicide
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
SOLVENT SOLUBILITY
REFRACTIVE INDEX
Stable under ordinary conditions.
EXTERNAL LINKS & GENERAL DESCRIPTION
Wikipedia Linking - Paclobutrazol
Google Scholar Search - Paclobutrazol
Drug Information Portal (U.S. National Library of Medicine) - Paclobutrazol
PubChem Compound Summary - Paclobutrazol
http://www.pesticideinfo.org/ - PaclobutrazolKEGG (Kyoto Encyclopedia of Genes and Genomes) - Paclobutrazol
http://www.ebi.ac.uk/ - Paclobutrazol
http://www.ncbi.nlm.nih.gov/ - Paclobutrazol
Local:
Paclobutrazol is a triazole type plant growth retardant which blocks
gibberellin biosynthesis and are involved in reducing abscisic
acid, ethylene and indole-3-acetic acid while increasing cytokinin
levels. It promotes fruit set in the states of inhibited development . It is also known that it protect plants against abiotic stresses. Uniconazol is another triazole type plant growth retardant. Though each plant hormone evokes many different specific biochemical, physiological, or morphological responses, the effects of different hormones overlap and may be stimulatory or inhibitory to regulate plant development and growth. Paclobutrazol it can be classified as the growth retardants, which can be sub-classified as gibberellin biosynthesis inhibitors or compounds which are not involved in
inhibiting gibberellin biosynthesis. [abscisic acid, cimetacarb, daminozide, dikegulac, maleic hydrazide, mefluidide, and morphologically active substances (morphactins: chlorfluren, chlorflurenol, dichlorflurenol, flurenol)].
Some commercial growth retardants are;
- Abscisic acid [(2Z,4E)-5-[(1S)-1-hydroxy-2,6,6-trimethyl-4-oxo-2-cyclohexen-1-yl]-3-methyl-2,4- pentadienoic acid, CAS RN: 21293-29-8]
- Ancymidol [alpha-cyclopropyl-α-(4-methoxyphenyl)-5-pyrimidinemethanol, CAS RN: 12771-68-5]
- Butralin [4-(1,1-dimethylethyl)-N-(1-methylpropyl)-2,6-dinitrobenzenamine, CAS RN: 33629-47-9]
- Carbaryl [1-naphthalenyl methylcarbamate, CAS RN: 63-25-2]
- Chlorfluren [2-chloro-9H-fluorene-9-carboxylic acid, CAS RN: 24539-66-0]
- Chlorflurenol 2-chloro-9-hydroxy-9H-fluorene-9-carboxylic acid, CAS RN: 2464-37-1]
- Chlormequat 2-chloro-N,N,N-trimethylethanaminium CAS RN.:7003-89-6
- Chlorpropham [1-methylethyl (3-chlorophenyl)carbamate, CAS RN: 101-21-3]
- Daminozide butanedioic acid mono(2,2-dimethylhydrazide) CAS RN.:1596-84-5
- Dichlorflurenol [2,7-dichloro-9-hydroxy-9H-fluorene-9-carboxylic acid, CAS RN: 69622-79-3]
- Dikegulac [2,3:4,6-bis-O-(1-methylethylidene)-α-L-xylo-2-hexulofuranosonic acid, CAS RN: 18467-77-1]
- Flumetralin [2-chloro-N-[2,6-dinitro-4-(trifluoromethyl)phenyl]-N-ethyl-6- fluorobenzenemethanamine, CAS RN: 62924-70-3]
- Fluoridamid [N-[4-methyl-3-[[(trifluoromethyl)sulfonyl]amino]phenyl]acetamide, CAS RN: 47000-92-0]
- Flurenol [9-hydroxy-9H-fluorene-9-carboxylic acid, CAS RN: 467-69-6]
- Flurprimidol [alpha-(1-methylethyl)-α-[4-(trifluoromethoxy)phenyl]-5-pyrimidinemethanol, CAS RN: 56425-91-3]
- Fosamine [ethyl hydrogen (aminocarbonyl)phosphonate, CAS RN: 59682-52-9]
- Glyphosine [N,N-bis(phosphonomethyl)glycine, CAS RN: 2439-99-8]
- Isopyrimol [alpha-(4-chlorophenyl)-α-(1-methylethyl)-5-pyrimidinemethanol, CAS RN: 55283-69-7]
- Jasmonic acid [(1R,2R)-3-oxo-2-(2Z)-2-pentenylcyclopentaneacetic acid, CAS RN: 6894-38-8]
- Maleic hydrazide [1,2-dihydro-3,6-pyridazinedione, CAS RN: 123-33-1]
- Mefluidide [N-[2,4-dimethyl-5-[[(trifluoromethyl)sulfonyl]amino]phenyl]acetamide, CAS RN: 53780-34-0]
- Mepiquat [1,1-dimethylpiperidinium, CAS RN: 15302-91-7]
- Paclobutrazol [(±)-(R',R')-beta-[(4-chlorophenyl)methyl]-alpha-(1,1-dimethylethyl)-1H-1,2,4-triazole-1- ethanol, CAS RN: 76738-62-0]
- Piproctanyl [1-(3,7-dimethyloctyl)-1-(2-propenyl)piperidinium, CAS RN: 69309-47-3]
- Prohydrojasmon [ (±)-Propyl (1R,2R)-3-oxo-2-pentylcyclopentaneacetate, CAS RN: 158474-72-7]
- Propham [1-methylethyl phenylcarbamate, CAS RN: 122-42-9]
- Tetcyclacis [ (±)-(3aR,4R,4aS,6aR,7R,7aS)-1-(4-chlorophenyl)-3a,4,4a,6a,7,7a- hexahydro-4,7-methano-1H-[1,2]diazeto[3,4-f] benzotriazole, CAS RN: 77788-21-7]
- 2,3,5-Triiodobenzoic acid [CAS RN: 88-82-4]
- Uniconazole [ (betaE)-beta-[(4-chlorophenyl)methylene]-alpha-(1,1-dimethylethyl)-1H-1,2,4- triazole-1-ethanol, CAS RN: 83657-22-1]
APPEARANCE
ASSAY
95.0% min
ACIDITY
0.5% max
Loss on drying
1.0% max
INSOLUBLES
0.5% max (in acetone)
Hazard Symbols: XN, Risk Phrases: 22, Safety Phrase: 36
GENERAL DESCRIPTION OF PLANT HORMONE
- Auxins
- 4-Chlorophenoxyacetic acid (CAS RN: 122-88-3)
- (2,4-Dichlorophenoxy)acetic acid (CAS RN: 94-75-7)
- 4-(2,4-Dichlorophenoxy)butyric acid (CAS RN: 94-82-6)
- Tris[2-(2,4-Dichlorophenoxy)ethyl] phosphite (CAS RN: 94-84-8)
- 2-(2,4-Dichlorophenoxy)propanoic acid (CAS RN: 120-36-5)
- 2-(2,4,5-trichlorophenoxy)propanoic acid (CAS RN: 93-72-1)
- Indole-3-acetic acid (CAS RN: 87-51-4)
- Indole-3-butyric acid (CAS RN: 133-32-4)
- 1-Naphthaleneacetamide (CAS RN: 86-86-2)
- 1-Naphthaleneacetic acid (CAS RN: 86-87-3)
- 1-Naphthol (CAS RN: 90-15-3)
- Naphthoxy acetic acid (CAS RN: 120-23-0)
- Naphthenic acid, inorganic salts (potassium, sodium)
- (2,4,5-Trichlorophenoxy) Acetic acid (CAS RN: 93-76-5)
- Antiauxins
- Clofibric acid (CAS RN: 882-09-7)
- 2,3,5-Triiodobenzoic acid (CAS RN: 88-82-4)
Cytokinin is a N6-substituted adenines acting as phytohormones such as kinetin, zeatin, 6-isopentenyladenine, benzyl adenine. The principal functions are stimulate cell division in concert with auxin (cytokinesis) and influence the pathway of tissue differentiation (organogenesis). 6-Benzylaminopurine is the first generation synthetic cytokinin which elicits plant growth and development responses setting blossoms and stimulating fruit richness by stimulating cell division. Active cytokinin ingredients include:
- Adenine (CAS RN: 73-24-5)
- Adenine Hemisulfate salt (CAS RN: 321-30-2)
- 6-Benzylaminopurine (CAS RN: 1214-39-7(base), 162714-86-5(HCl)
- N-Benzyl-9-(2-tetrahydropyranyl)adenine (CAS RN: 2312-73-4)
- N-(2-Chloro-4-pyridyl)-N'-phenylurea (CAS RN: 68157-60-8)
- 6-(gamma,gamma-Dimethylallylamino)purine (CAS RN: 2365-40-4)
- 1,3-Diphenylurea (CAS RN: 102-07-8)
- Kinetin (CAS RN: 525-79-1 (base), 177966-68-6 (HCl)
- 1-Phenyl-3-(1,2,3-thiadiazol-5-yl) Urea (CAS RN: 51707-55-2)
- Zeatin (CAS RN: 13114-27-7)
- trans-Zeatin (CAS RN: 1637-39-4 (base), 6025-81-6 (HCl))
- trans-Zeatin riboside (CAS RN: 6025-53-2)
Other Plant Growth Regulators include:
- Abscisic acid (CAS RN: 21293-29-8)
- Ancymidol (CAS RN: 12771-68-5)
- Chlorocholine chloride (CAS RN: 999-81-5)
- Daminozide (CAS RN: 1596-84-5)
- 3,6-Dichloro-o-anisic acid (CAS RN: 1918-00-9)
- Gibberellic acid (CAS RN: 77-06-5)
- Gibberellic acid Potassium salt (CAS RN: 125-67-7)
- Gibberellin A4 (CAS RN: 468-44-0 ) and other gibberellins (more than 110 gibberellins are known)
- Glyphosate (CAS RN: 1071-83-6)
- Jasmonic acid (CAS RN: 3572-66-5)
- 1,3,5-Trihydroxybenzene (CAS RN: 108-73-6)