| CAS
NO. |
100-63-0
(Base), 59-88-1 (HCl) |
|
| EINECS NO. |
202-873-5 |
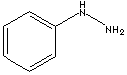
| FORMULA |
C6H5NHNH2 |
| MOL
WT. |
108.14
|
|
H.S.
CODE
|
2928.00 |
|
TOXICITY
|
Oral rat LD50: 188 mg/kg |
| SYNONYMS |
Hydrazinobenzene; Phenyldiazane; Fenilidrazina (Italian); |
| Monophenylhydrazine;
Fenylhydrazine (Dutch); Phenylhydrazin (German); |
| INGREDIENTS |
|
|
CLASSIFICATION
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
yellow to pale brown
oily liquid. |
| MELTING POINT |
19.5
C |
| BOILING
POINT |
243.5
C |
| SPECIFIC GRAVITY |
1.099
|
| SOLUBILITY
IN WATER |
sparingly soluble |
|
SOLVENT SOLUBILITY
|
miscible with other organic solvents |
| pH |
|
| VAPOR DENSITY |
3.7 |
|
REFRACTIVE
INDEX
|
1.6080 |
| AUTOIGNITION |
174
C
|
| NFPA RATINGS |
Health: 3; Flammability: 2; Reactivity: 0 |
| FLASH
POINT |
88
C
|
| STABILITY |
Stable under ordinary conditions. Light
sensitive |
|
APPLICATIONS
|
Phenylhydrazine
is the starting material to produce heterocycle
indoles. This indole forming reaction with an aldehyde or ketone under acidic
conditions is called
Fischer indole synthesis.
A (substituted). Other reactions include;
- Bartoli reaction
- Bischler-Möhlau reaction
- Fischer reaction
- Gassman reaction
- Hemetsberger reaction
- Larock
reaction
- Leimgruber-Batcho
reaction
- Madelung reaction
- Martinet dioxreaction
- Nenitzescu reaction
- Reissert
reaction
Phenylhydrazine is used
as a chemical
intermediate in the pharmaceutica1ls particularly of tryptamine drugs used in the treatment of migraine and cluster headaches.
It
is used to prepare agrochemical, and other chemical. It
is used to detect sugars and aldehydes
in analytical chemistry.
|
| SALES
SPECIFICATION |
|
APPEARANCE
|
yellow to pale brown
oily liquid |
|
CONTENT
|
99.0%
min or 85.0% min
|
|
MELTING
POINT
|
19
C
|
|
ASH
|
0.5%
max
|
| TRANSPORTATION |
| PACKING |
200kgs
in drum
|
| HAZARD CLASS |
6.1(Packing
group : II) |
| UN
NO. |
2572 |
| GENERAL
DESCRIPTION OF HYDRAZINE
|
Hydrazine (anhydrous),
H2NNH2,
is a clear, fuming, corrosive liquid with an ammonia-like
odor; melting at 1.4 C, boiling at 113.5 C, specific gravity 1.011. It is very
soluble in water and soluble in alcohol. It decomposes on heating or exposure to
UV to form ammonia, hydrogen, and nitrogen, which may be explosive with a blue
flame when catalysed by metal oxides and some metals such as platinum or Raney
nickel. It is prepared from ammonia with chloramine in the presence of glue or
gelatin (to inhibit decomposition of the hydrazine by unreacted oxidants) as the
hydrate form usually (100% monohydrate contains 64% by weight hydrazine).
Hydrazine is also prepared from sodium hypochlorite with urea in the presence of
glue or gelatin. Botht ammonia and amines are nitrogen nucleophiles which
donate electrons (they are Lewis bases). But hydrazine (diamine) has much
stronger nucleophilicity which makes it more reactive than ammonia. Hydrazine
has dibasic and very reactive properties. Hydrazine is used as a component in
jet fuels because it produce a large amount of heat when burned. It is less flammable and less volatile
than hydrocarbon fuels. It is relatively environmentally friendly because
they degrade quickly in the environment. Hydrazine is used as an oxygen scavenger for water boiler
feed and heating systems to prevent corrosion damage. Hydrazine is used as a
reducing agent for the recovery of precious metals. It is used as a
polymerization catalyst and a chain extender in urethane coatings. It, or a
derivative thereof, is versatile intermediate. They have active applications in
organic synthesis for agrochemicals, pharmaceuticals, photographic, heat
stabilizers, polymerization catalysts, flame-retardants, blowing agents for
plastics, explosives, and dyes. Recently, hydrazine is applied to LCD (liquid
crystal displays) as the fuel to make faster thin-film transistors. Hydrazone is a
compound containing the group -NH·N:C-. It is formed from a condensation
reaction aldehydes or ketones with hydrazines (commonly phenylhydrazine). It is
used as an exotic fuel. Aromatic hydrazones are used to form indole by a ring
closure reaction (Fischer synthesis). Hydrazones and hydrazines are converted to
aldehydes and ketones to corresponding hydrocarbons by heating the carbonyl
compound with sodium ethoxide (Wolf-Kishner reduction). Azide
contains the group -N3
represented as a resonance hybrid of two structures,
-N-N-��N+
�� -N=N+=N-.
Organic azides are compounds replaced by a hydrocarbon group as in alkyl or aryl
from hydrazoic acid (HN3)and have general formula RN3. Acyl azide is a compound in
which the hydroxy group of a carboxylic acid is replaced by the azido group
(HN3). Hydrazide is an acyl hydrazine. Acyl (-CO) is an organic radical formed by
removal of a hydroxyl group from an organic acid (carboxyl group). Organic azides are useful for the synthesis of target compounds. They act
as electrophiles on the nitrogen attached to the carbon and have
electron-donating character for the neighboring carbon.
|
