PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
GENERAL DESCRIPTION OF INOSITOL
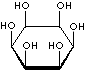
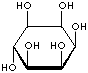
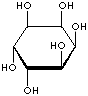
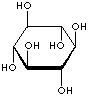
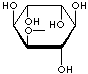
Inositol ,chemically hexahydroxycylohexane, is any of nine stereoisomeric alcohols that closely resemble glucose in structure. It is a constituent of many cell phosphoglycerides. Meso- or myoinositol, named for its presence in muscle tissue, is biologically the important isomer. Myo-Inositol is the precursor in the phosphatidylinositol cycle, a source of two second messengers (diacylglycerol and inositol triphosphate). Inositols and their phosphates lack a hydrolytically labile glycosidic linkage and are stable to degradative enzymes in vivo. They have been used in the stable insulin mediators, inhibitors, and modulators. It is known that Inositols are effective in relieving symptoms of depression. Though inositols are not regarded as an essential nutrient in humans, they are sometimes classified as a member of the vitamin B complex (thiamine, riboflavin, niacin, pantothenic acid, biotin, pyridoxine, folic acid, inositol, and vitamin B12). Inositol is essential for the growth of some yeasts and fungi.
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
Stable under ordinary conditions
GENERAL DESCRIPTION & APPLICATIONS
APPEARANCE
ASSAY
98.0% min
OPTICAL ROTATION
+60 ±1° (C=1 in water)
HEAVY METALS
5ppm max
LOSS ON DRYING
1.0% max
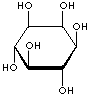
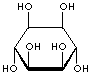
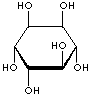
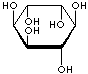
INOSITOL ISOMERS
|
|
|
|
|
|
cis-INOSITOL |
epi-INOSITOL |
allo-INOSITOL |
neo-INOSITOL |
|
|
|
|
|
|
myo-INOSITOL |
muco-INOSITOL |
scyllo-INOSITOL |
L-(-)-chiro-INOSITOL |
|
|
|
|
|
|
D-(+)-chiro-INOSITOL |
|
|
|