PRODUCT IDENTIFICATION
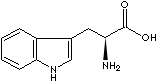
200-795-6
H.S. CODE
TOXICITY
CLASSIFICATION
Amino acid, Antidepressive, Psychotropic
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
white crystals
1.34
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
Stable under ordinary conditions. Moisture, light sensitive.
EXTERNAL LINKS & GENERAL DESCRIPTION
Drug Information Portal (U.S. National Library of Medicine) - Tryptophan
PubChem Compound Summary - L-Tryptophan
Drug Bank - Tryptophan
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Tryptophan
http://www.ebi.ac.uk/chebi/ - Tryptophan
http://www.ncbi.nlm.nih.gov/ - Tryptophan
Human Metabolome Database - Tryptophan
http://www.iscid.org/
Tryptophan
is an amino acid; one of the 20 found in the basic genetic code:
codon UGG. It is essential in human nutrition, and may have significant
effects on the human brain. Tryptophan is a precursor for serotonin,
melatonin, and niacin. In those who cannot metabolize it properly,
it's possible that tryptophan is a cause of schizophrenia. Improperly
metabolized, its waste products are toxic to the brain and cause
hallucinations and delusions. However, it's also been found to aid
schizophrenic patients. Tryptophan is produced by the human body,
but can also be found in many sources of dietary protein, including
chocolate, oats, bananas, dried dates, cottage cheese, red meat,
fish, milk, peanuts, and most famously turkey. According to a widespread
myth, when the tryptophan in turkey is consumed, it is supposed
to make the person consuming it very sleepy within a matter of hours.
Whether this is fact or not is doubtful, especially the uniqueness
of turkey. Still, tryptophan supplements have been used as a safe
and somewhat effective sleep aid. This effect is probably because
of its ability to increase brain levels of serotonin and melatonin.
Serotonin is known for its calming effects, and melatonin, secreted
by the pineal gland when dark or low light levels predominate, induces
slumber. According to clinical research, tryptophan does act reasonably
well as a sleep inducer, an antidepressant, and an augmentor of
antidepressants. Chronic pain and reduction of negative extreme
behaviors (impulsivity, mania, addiction, etc.) has also been indicated
by researchers. And it may be effective in treating seasonal-affective
disorder, or SAD. However, due to an outbreak of eosinophilia-myalgia
syndrome (a sometimes-deadly autoimmune disease) traced to taking
tryptophan from Japan, tryptophan supplements were banned by numerous
companies. The problem was ultimately identified as probably a contaminant
and not tryptophan at all, but the health agencies of many countries
still remain leery of tryptophan. Despite this, psychiatrists continue
to prescribe tryptophan as an antidepressant by itself or, more
often, as an augmentor for other antidepressants in patients who
are unresponsive to antidepressants.
Local:
Amino Acid is any of the organic compounds in which one (or more ) amino group
(-NH2) and one (or more ) carboxylic acid group (-COOH) are both present
with general formula R-CH(NH2)COOH
containing carbon, hydrogen, oxygen, nitrogen, and in certain cases sulfur atoms. Two groups attached to the same carbon
(called the alpha-carbon atom at the end of the compound) are polymerized to form
peptides and proteins. The amine group is protonated to form -NH3+ at low pH. The
carboxylic acid group is deprotonated to form -CO2- at high pH. The carbon atom
in the carboxyl group of one amino acid binds covalently to the nitrogen atom in
the amino group of another amino acid to form a peptide bond with the release of
a water molecule. Proteins are synthesized through the covalent chemical
polypeptide bonds. The sequence of these amino acids in the protein polypeptides
determines the shape, properties, and hence biological role of the protein that
function as chemical messengers and as intermediates in metabolism. Proteins are
composed of various proportions of about 20 commonly occurring amino acids.
Plants or other biological systems can synthesize amino acids from simple
inorganic compounds, but animals rely on adequate supplies in their diet. More
than 100 common amino acids occur in plants or in other microorganic systems.
The 20 amino acids commonly found in animals are Alanine, Arginine, Asparagine,
Aspartic Acid, Cysteine, Glutamic Acid, Glutamine, Glycine, Histidine,
Isoleucine, Leucine, Lysine, Methionine, Phenylalanine, Proline, Serine,
Threonine, Tryptophan, Tyrosine, and Valine. Many of the amino acids can be
synthesized in the human or animal body from other cellular metabolites when needed (called Non-essential Amino
Acids). Animals are not able to synthesize some amino acids necessary in
metabolism in sufficient quantities. It must therefore be present in the diet
(called Essential Amino Acids). In man, these essential amino acids are
Arginine, Histidine, Isoleucine, Leucine, Lysine, Methionine, Phenylalanine,
Threonine, Tryptophan and Valine. Tryptophan is formed from proteins during digestion by the action of proteolytic
enzymes and is necessary for normal growth and development. Branched amino-acids (Valine, Isoleucine and Leucine ) with
tryptophan
and phenylalanine (aromatic side chain amino acids) contribute to the structure
of protein by the tendency of its side chain composed only of carbon and
hydrogen to participate in hydrophobic interactions which determines the
tertiary structure of the peptide chain. Infants
require greater amounts of tryptophan than adults. Only the l-stereoisomer is
biologically active form in mammalian protein. Tryptophan is also a precursor
for serotonin (a neurotransmitter), melatonin (a neurohormone) and niacin (a
vitamin of the B complex).
APPEARANCE
white crystals
98.0 - 101.0%
OPTICAL ROTATION
-29..5° ~ -33.0° (c=1 in water)
LOSS ON DRYING
0.3% max
RESIDUE ON IGNITION
0.1% max
NH4
0.02% max
CHLORIDE
0.02% max
SULFATE
0.02% max
HEAVY METALS
10ppm max
ARSENIC
1ppm max
GENERAL PROPERTIES OF AMINO ACIDS
|
Amino Acid |
Abbreviation |
Formula (Mol WT) |
pK1 |
pK2 |
pKR |
pI |
Hydropathy Index |
|
|
3-Letters |
1-Letter |
-COOH |
-NH3+ |
R group |
||||
| Alanine |
Ala |
A |
C3H7NO2 (89.09) |
2.34 |
9.69 |
- |
6.00 |
1.8 |
| Arginine |
Arg |
R |
C6H14N4O2(174.20) |
2.17 |
9.04 |
12.48 |
10.76 |
-4.5 |
| Asparagine |
Asn |
N |
C4H8N2O3(132.12) |
2.02 |
8.80 |
- |
5.41 |
-3.5 |
| Aspartic Acid |
Asp |
D |
C4H7NO4(133.10) |
1.88 |
9.60 |
3.65 |
2.77 |
-3.5 |
| Cysteine |
Cys |
C |
C3H7NO2S(240.30) |
1.96 |
10.128 |
8.18 |
5.07 |
2.5 |
| Glutamic Acid |
Glu |
E |
C5H9NO4(147.13) |
2.19 |
9.67 |
4.25 |
3.22 |
-3.5 |
| Glutamine |
Gln |
Q |
C5H10N2O3(146.15) |
2.17 |
9.13 |
- |
5.65 |
-3.5 |
| Glycine |
Gly |
G |
C2H5O2(75.07) |
2.34 |
9.60 |
- |
5.97 |
-0.4 |
| Histidine |
His |
H |
C6H9N3O2(155.16) |
1.82 |
9.17 |
6.00 |
7.59 |
-3.2 |
| Isoleucine |
Ile |
I |
C6H13NO2(131.18) |
2.36 |
9.60 |
- |
6.02 |
4.5 |
| Leucine |
Leu |
L |
C6H13NO2(131.18) |
2.36 |
9.60 |
- |
5.98 |
3.8 |
| Lysine |
Lys |
K |
C6H14N2O2(146.19) |
2.18 |
8.95 |
10.53 |
9.74 |
-3.9 |
| Methionine |
Met |
M |
C5H11NO2S(149.21) |
2.28 |
9.21 |
- |
5.74 |
1.9 |
| Phenylalanine |
Phe |
F |
C9H11NO2(165.19) |
1.83 |
9.13 |
- |
5.48 |
2.8 |
| Proline |
Pro |
P |
C5H9NO2(115.13) |
1.99 |
10.60 |
- |
6.30 |
1.6 |
| Serine |
Ser |
S |
C3H7NO3(105.19) |
2.21 |
9.15 |
- |
5.58 |
-0.8 |
| Threonine |
Thr |
T |
C4H9NO3(119.12) |
2.09 |
9.10 |
- |
5.60 |
-0.7 |
| Tryptophan |
Trp |
W |
C11H10N2O2(204.23) |
2.83 |
9.39 |
- |
5.89 |
-0.9 |
| Tyrosine |
Tyr |
Y |
C9H11NO3(181.19) |
2.20 |
9.11 |
10.07 |
5.66 |
-1.3 |
| Valine |
Val |
V |
C5H11NO2(117.15) |
2.32 |
9.62 |
- |
5.96 |
4.2 |