PRODUCT IDENTIFICATION
207-984-2
H.S. CODE
TOXICITY
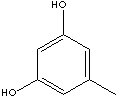
c1(cc(cc(c1)O)O)C
CLASSIFICATION
Alkylresorcinol
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
EXTERNAL LINKS & GENERAL DESCRIPTION
USA.gov - Orcinol
Wikipedia Linking - Orcinol
Google Scholar Search - Orcinol
U.S. National Library of Medicine - Orcinol
PubChem Compound Summary - Orcinol
Drug Bank - Orcinol
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Orcinol
ChEBI (http://www.ebi.ac.uk/chebi) - Orcinol
NCBI (http://www.ncbi.nlm.nih.gov/) - Orcinol
Material Safety Data Sheet - Orcinol
EPA - Substance Registry Services - Orcinol
Local:
GENERAL DESCRIPTION OF RESORCINOL: There are three isomeric compounds of dihydroxybenzene molecule structure, which all have traditional names respectively. The ortho (1,2) isomer is called catechol (Also known as catechin; pyrocatechol; pyrocatechuic acid), which forms clear crystals used as a photographic developer in solution and as a starting material to produce synthetic catecholamines which have important physiological effects as neurotransmitters and hormonesany (such as epinephrine, adrenaline, norepinephrine, and dopamine). The meta (1,3) isomer is resorcinol (also known as resorcin), which forms clear needle crystals used in the production of diazo dyes and plasticizers. It is produced by sulfonating benzene with fuming sulfuric acid and fusing the resulting benzenedisulfonic acid with caustic soda. Resorcinol is used in resins as an UV absorber. It is used in manufacturing fluorescent and leather dyes and adhesives. Reaction with formaldehyde produces resins (resorcinol formaldehyde resins) used to make rayon and nylon. It is used as a pharmaceutical to treat acne and other greasy skin conditions in combination with other acne treatments such as sulfur. It is used as an anti-dandruff agent in shampoo and sunscreen cosmetics. It is also used as a chemical intermediate to synthesis pharmaceuticals and other organic compounds. The para (1,4) isomer is hydroquinone (also known as quinol), which forms clear prisms used as a photographic reducer and developer (except in color film). It is formed in large quantities by chemical reduction of benzoquinone. This compound is a general-purpose inhibitor, stabilizer, antioxidant, and intermediate. One of the major uses of hydroquinone is as an intermediate to make other inhibitors, stabilizers, antioxidants, agricultural chemicals, and dyes. Resorcinol and its derivatives are used in resins as UV absorbers. They are used in manufacturing fluorescent and leather dyes and adhesives (resorcinol formaldehyde resins). They are used as pharmaceuticals to treat acne and other greasy skin conditions in combination with other acne treatments such s sulfur. They are used as an anti-dandruff agent in shampoo and sunscreen cosmetics.
APPLICATIONS: Alkyl substituted resorcinols are used in manufacturing photoactive and novolac resins for electronics industry, UV protectors, and stabilizers and antioxidants for plastics and rubbers. They are also used in analgesics, food additives, fragrances, biocides, fungicides, dyes for pharmaceuticals and food additive industry. It is believed that 5-methylresorcinol (5th position methyl ester) is low toxic compared to other resorcinols.
APPEARANCE
98.5% min
HAZARD OVERVIEW
GHS (Globally Harmonised System) Classification: Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. Hazard statements: Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation.
GHS
Warning
PICTOGRAMS

HAZARD STATEMENTS
H302-H315-H319-H335
P STATEMENTS
P261-P305 + P351 + P338
![]()
RISK PHRASES
22-36/37/38
SAFETY PHRASES
22-26-36