|
PYRIDINE,
also called azabenzene and azine, is a heterocyclic
aromatic tertiary amine characterized by a six-membered ring structure
composed of five carbon atoms and one carbon-hydrogen unit in
the benzene ring being replaced with a nitrogen molecule. The simplest member of the
pyridine family is pyridine itself. It is colorless, flammable, toxic liquid with a unpleasant
odor, miscible with
water and with most organic solvents, boils at 115 C. Its aqueous solution is slightly alkaline.
It is incompatible and reactive with strong oxidizers and strong
acids, and reacts violently with chlorosulfonic acid, maleic anhydride, oleum,
perchromates, B-propiolactone, formamide, chromium trioxide, and sulfuric acid.
Liquid pyridine easily evaporates into the air. If it is released to the air, it
may take several months to years until it breaks down into other compounds.
It is made from crude coal tar or from other chemicals
based on acetaldehyde and ammonia. It is used as a
solvent as a denaturant for alcohol and is added to ethyl
alcohol to make it unfit for drinking. It is a starting
material in the synthesis of other compounds used as an intermediate in making insecticides,
herbicides, medicines,
vitamins, food flavorings, dyes, rubber chemiclas, and adhesives such as sulfapyridine,
pyribenzamine, pyrilamine and piperidine. Compounds not made from pyridine but containing
its ring structure include niacin and pyridoxal; isoniazid, nicotine and several other nitrogenous plant
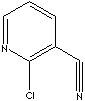
products. Chloronicotinonitrile
is an intermediate
for pharmaceuticals and agrochemicals. |
|
Nitrile is an organic compounds containing cyano group (-C��N, containing
trivalent nitrogen) which is attached to one carbon atom with the general
formula RC��N. Their names are corresponding to carboxylic acids by changing '-ic
acid' to the suffix, '-onitrile' which denotes only the ��N atom (triply bound)
excluding the carbon atom attached to it, or the suffix, '-carbonitrile' where
the carbon atom in the -CN is included, whichever preserves a single letter O.
Examples are acetonitrile from acetic acid and benzonitrile from benzoic acid.
The prefix, 'cyano-' is used as an alternative naming system to indicate the
presence of a nitrile group in a molecule for the compounds of salts and
organic derivatives of hydrogen cyanide (HC��N). Isocyanides are salts and hydrocarbyl
derivatives from the isomer, HN+��C-.
Sodium cyanide, NaCN; potassium
cyanide, KCN; calcium cyanide, Ca(CN)2; and hydrocyanic (or prussic) acid, HCN
are examples. Chemically, the simple inorganic cyanides resemble chlorides in
many ways. Organic nitriles act as solvents and are reacted further for various application including;
·
Extraction solvent for fatty acids,
oils and unsaturated hydrocarbons
· Solvent for spinning and casting and
extractive distillation based on its selective miscibility with organic
compounds.
· Removing agent of colouring matters and aromatic
alcohols
· Non-aqueous solvent for titrations and for inorganic
salts
· Recrystallization of steroids
·
Parent compound for organic
synthesis
· Solvent or chemical intermediate in biochemistry ( pesticide
sequencing and DNA synthesis)
·
High-pressure liquid chromatographic
analysis
· Catalyst and component of transition-metal complex
catalysts
· Stabilizer for chlorinated
solvents
· Chemical intermediate and solvent for perfumes and
pharmaceuticals
|