2-DEOXYURIDINE
PRODUCT IDENTIFICATION
951-78-0
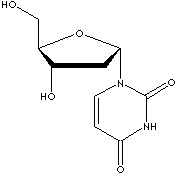
C9H12N2O5
228.20
H.S. CODE
TOXICITY
CLASSIFICATION
EXTRA NOTES
2��-Deoxyuridine (dU) is frequently halogenated to create thymidine analogues useful for studies of DNA synthesis and degradation mechanisms. Derivatized 2��-Deoxyuridines used as labeling substrates include chloro-2��-deoxyuridine (CldU), bromodeoxyuridine (BrdU) and/or iododeoxyuridine (IdU). Other useful analogues of 2��-deoxyuridine include 5-ethynyl-2��-deoxyuridine (DdU) and 5-hydroxymethyl-2��-deoxyuridine (HmdU).
PHYSICAL AND CHEMICAL PROPERTIES
white to off-white crystalline powder
163 - 167 C
REFRACTIVE INDEX
Stable under ordinary conditions
EXTERNAL LINKS & GENERAL DESCRIPTION
Drug Information Portal (U.S. National Library of Medicine) - Deoxyuridine
PubChem Compound Summary - Deoxyuridine
Drug Bank - Deoxyuridine
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Deoxyuridine
Local:
Ribose
is a pentose (five-carbon sugar) that is a component
of the ribonucleic acid (RNA), where it alternates with
phosphate groups to form the 'back-bone' of the RNA
polymer and binds to nitrogenous bases. Ribose phosphates
are components of the nucleotide coenzymes and are utilized
by microorganisms in the synthesis of the amino acid
histidine. Its close relative, deoxyribose, is a constituent
of deoxyribonucleic acid (DNA), where it alternates
with phosphate groups to form the 'back-bone' of the
DNA polymer and binds to nitrogenous bases. The presence
of deoxyribose instead of ribose is one difference between
DNA and RNA. Ribose has one more oxygen atom in its
molecule than deoxyribose. Ribose has a five member
ring composed of four carbon atoms and one oxygen. Hydroxyl
groups are attached to three of the carbons. The other
carbon and a hydroxyl group are attached to one of the
carbon atoms adjacent to the oxygen. In deoxyribose,
the carbon furthest from the attached carbon is stripped
of the oxygen atom in what would be a hydroxyl group
in ribose. The sugar (ribose or deoxyribose) molecules
in the nucleic acid are all oriented in the same direction.
Their carbon atoms are numbered: the 5' carbon atom
is always on the side of the sugar molecule that faces
the leading end, while the 3' carbon atom always faces
the tail end. Nucleotide is the structural unit of a
nucleic acid. A nucleotide consists of either a nitrogenous
heterocyclic base (purine or pyrimidine) , a pentose
sugar (ribose or deoxyribose) and a phosphate group
attached at the 5' position on the sugar. A nucleoside
consists of only a pentose sugar linked to a purine
or pyrimidine base, without a phosphate group. Purine
bases are Adenine, Guanine and Hypoxanthine (examples
of purine nucleosides are Adenosine, 2'-Deoxyadenosine,
Guanosine, 2'-Deoxyguanosine, Inosine, 2'-Deoxyinosine).
Pyrimidine bases are Cytosine, Thymine, and Uracil (examples
of pyrimidine nucleosides are Cytidine, 2'-Deoxyguanosine,
5-Methyluridine, 2'-Deoxy-5-Methyluridine, Uridine,
2'-Deoxyuridine). The nucleoside derivatives are involved
in important functions in cellular metabolism and are
used to synthesize enzyme inhibitors, antiviral agents,
and anticancer agents.
- Urasil: a pyrimidine base
- Uridinea pyrimidine nucleoside
- Uridine Monophosphate (UMP, Uridylic acid): a nucleotide, the 5'-phosphate of uridine; a component of ribonucleic acid.
- Uridine Diphosphate (UDP): a nucleotide, the 5'-pyrophosphate of uridine; acting as the chief transferring coenzyme in carbohydrate metabolism; acting as a carrier of hexoses, hexosamines, and hexuronic acids which are intermediates in the metabolism.
- Uridine Triphosphate (UTP): a nucleotide, the 5'-triphosphate of uridine; acting as a precursor in the synthesis of ribonucleic acid and of UDP-linked intermediates.
- Deoxyuridine Monophosphate (dUMP): a nucleotide, the 5'-phosphate of deoxyuridine; an intermediate in the synthesis of deoxythymidine triphosphate. (deoxy-, also called desoxy, is a prefix for the designation of compounds which contain one less atom of oxygen than the reference substance).
- Deoxyuridine Diphosphate (dUDP): a nucleotide, the 5'-phosphate of deoxyuridine; an intermediate in the synthesis of deoxythymidine triphosphate.
- Deoxyuridine Triphosphate (dUTP): a nucleotide, the 5'-triphosphate of deoxyuridine; an intermediate in the synthesis of deoxyribonucleotides.
Chemically modified nucleotides substituted or attached by special chemical groups or elements are studied and used to inactivate the normal biological operation in the living organism and the function of important enzymes.
APPEARANCE
fine off-white crystalline powder
97.0 - 102.0 %
OPTICAL ROTATION
+ 28º ~ +31 º
MELTING POINT
163 - 167 C
Hazard Overview
Not known
RISK PHRASES
SAFETY PHRASES
24/25 Avoid contact with skin and eyes.