| CAS
NO. |
86-81-7 |
|
| EINECS NO. |
201-701-6 |
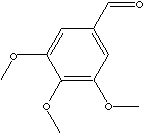
| FORMULA |
(CH3O)3C6H2CHO |
| MOL
WT. |
196.21 |
|
H.S.
CODE
|
2912.49 |
|
TOXICITY
|
Bird - wild LD50 (Oral):
422 mg/kg |
| SYNONYMS |
TMBA; |
| SMILES |
c1(c(cc(C=O)cc1OC)OC)OC |
|
CLASSIFICATION
|
Aldehyde |
|
PHYSICAL AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
white crystal |
| MELTING POINT |
73 - 75 C |
| BOILING
POINT |
|
| SPECIFIC GRAVITY |
|
| SOLUBILITY
IN WATER |
1490 mg/l at 25 C |
| SOLVENT
SOLUBILITY |
|
| pH |
|
| VAPOR DENSITY |
|
| log Pow |
1.39
(Octanol-water) |
| VAPOR
PRESSURE |
1.13E-03 (mmHg at 25 C) |
|
HENRY'S LAW |
(atm-m3/mole at 25 C) |
| OH RATE |
(cm3/molecule-sec
at 25 C Atmospheric) |
|
AUTOIGNITION
|
|
|
NFPA RATINGS
|
Health: 1 Flammability: 0 Reactivity: 0 |
|
REFRACTIVE INDEX
|
|
| FLASH
POINT |
|
| STABILITY |
Stable under ordinary conditions |
|
GENERAL
DESCRIPTION & EXTERNAL LINKS
|
|
3,4,5-Trimethoxybenzaldehyde is used as an intermediate for the synthesis of pharmaceuticals especially for Trimethoprim used to treat various bacterial infections, esp. urinary tract pathogens in combination with sulfamethoxazole.
http://www.umich.edu/:Synthesis
of 3,4,5-Trimethoxybenzaldehyde - Introduction: (what makes your
target interesting?) This molecule is interesting to us because
it is a very versatile molecule. It has many different uses spanning
various different fields of life. One of the more important uses
for Trimethozybenzaldehyde is that it is used to make pharmaceutical
products. It is an intermediate step which is more economically
efficient than methods previously used. One of the important medications
that Trimethoxybenzaldehyde helps to make is Trimethoprim (an antibiotic
that interferes with the production of folic acid – helping to treat
numerous bacterial infections - mostly used for urinary tract
infections). In addition to its very important pharmaceutical use,
Trimethoxybenzaldehyde is also used in many other areas of life.
Trimethoxybenzaldehyde is essential in the world of the culinary
arts, as it is used to help create the flavoring compounds used
in not only cooking but in the flavoring of beverages as well. Also,
Trimethoxybenzaldehyde can help with the development of fragrances
used in perfumes and other air fresheners. In addition to the wide
range of uses that Trimethoxybenzaldehyde has, it can also
be used in the production of plastic additives.
http://www.chemcas.org/
Material
Safety Data Sheet
Search
Google
Scholar Search
|
| SALES
SPECIFICATION |
|
APPEARANCE
|
white crystal |
|
ASSAY
|
99.0% MIN |
|
MELTING POINT |
73 - 75 C |
|
WATER |
0.1% max
|
| TRANSPORTATION |
| PACKING |
|
| HAZARD CLASS |
3 (Packing Group: III ) |
| UN
NO. |
1993 |
| OTHER INFORMATION |
|
Hazard Symbols: XI, Risk Phrases: 10/36/37/38, Safety Phrases: 16/24/25 |
|
GENERAL DESCRIPTION OF BENZALDEHYDE
|
|
Benzaldehyde(also
called Benzenecarbonal) is the simplest representative of the aromatic aldehydes.
It is a colorless liquid aldehyde with a characteristic almond odor. It
boils at 180° C, is soluble in ethanol, but is insoluble in water. Benzaldehyde
is formed
by partial oxidation of benzyl alcohol and readily oxidized to benzoic acid and is converted to
addition products by hydrocyanic acid or sodium bisulfite. It is also prepared by
oxidation of toluene or benzyl chloride or by treating benzal chloride with an
alkali, e.g., sodium hydroxide.
It is used chiefly in the synthesis of other organic compounds, ranging from pharmaceuticals to plastic
additives
and benzaldehyde is an important intermediate for the processing of perfume and flavouring
compounds and in the preparation of
certain aniline dyes . It is the first step in the synthesis for fragrances.
It undergoes
simultaneous oxidation and reduction with alcoholic potassium hydroxide, giving
potassium benzoate and benzyl alcohol. It is
converted to benzoin with alcoholic potassium cyanide, with anhydrous sodium acetate and acetic anhydride, giving cinnamic acid. Compounds which do not have alpha-hydrogen atoms cannot form an enolate ion and do not undergo electrophilic alpha-substitution and aldol condensation. Aromatic aldehydes such as benzaldehyde and formaldehyde may undergo disproportionation in concentrated alkali (Cannizaro's reaction) one molecule of the aldehyde is reduced to the corresponding alcohol and another molecule is simultaneously oxidized to the salt of a carboxylic acid. The speed of the reaction depends on the substituents in the aromatic ring. Two different types of aldehydes (aromatic and aliphatic) can undergo crossing reaction to form fomaldehyde and aromatic alcohols. |
