PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
317 C
REFRACTIVE INDEX
NFPA RATINGS
AUTOIGNITION
FLASH POINT
150 C
GENERAL DESCRIPTION & APPLICATIONS
Picoline : Three structural isomers of methyl pyridines (alpha, beta, gamma- positions)
Lutidine : Six structural isomers of dimethyl pyridines (2,3-, ,24-, 2,5-, 2,6-, 3,4-, 3,5- positions)
Collidine : Three structural isomers of trimethyl pyridines (2,3,5-, 2,3,6-, 2,4,6- positions)
Pyrimidine: Pyridine alteration containing nitrogen atoms at positions 1 and 3
Piperidine: Hexahydropyridine (saturated form)
Nicotinic acid: pyridine-3-carboxylic acid
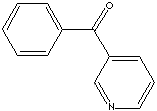
Pyridine and its derivatives are very important in industrial field as well as in bio chemistry. Nucleotide consist of either a nitrogenous heterocyclic base (purine or pyrimidine). Three major pyrimidines in living systems are cytosine, thymine, and uracil. Pyrimidine and its derivatives are biologically important components of nucleic acids (DNA, RNA) and coenzymes. Some pyridine system is active in the metabolism in the body. Certain nitrogenous plant products also have pyridine class compounds. They can be the parent compound of many drugs, including the barbiturates. Pyridine and its derivatives are used as solvents and starting material for the synthesis of target compounds such as insecticides, herbicides, medicines, vitamins, food flavorings, feed additives, dyes, rubber chemicals, explosives, disinfectants, and adhesives. Pyridine is also used as a denaturant for antifreeze mixtures, as a dyeing assistant in textiles and in fungicides. 3-Benzoylpyridine is used as an intermediate for pharmaceuticals and other organic synthesis.
APPEARANCE
ASSAY
98.0% min
MELTING POINT
0.2% max
IMPURITY
3-Benzylpyridine : 1.0% max
4-Benzoylpyridine: 0.5% max
4-Benzoylpyridine : 0.5% max
GENERAL DESCRIPTION OF KETONE