| CAS
NO. |
35303-76-5 |
|
| EINECS NO. |
252-501-0 |
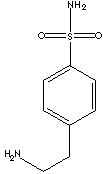
| FORMULA |
C8H12N2O2S |
| MOL
WT. |
200.26 |
|
H.S.
CODE
|
|
|
SMILES |
|
|
TOXICITY
|
|
| SYNONYMS |
4-(2'-Aminoethyl)benzenesulfonamide
|
| p-(2-Aminoethyl)benzenesulfonamide
|
|
CLASSIFICATION
|
BENZENESULFONAMIDE / |
|
PHYSICAL AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
white to off-white crystalline
powder |
| MELTING POINT |
142
- 149 C |
| BOILING
POINT |
|
| SPECIFIC GRAVITY |
|
| SOLUBILITY IN WATER |
|
| pH |
|
| VAPOR DENSITY |
|
| AUTOIGNITION |
|
| NFPA RATINGS |
|
| REFRACTIVE
INDEX
|
|
| FLASH
POINT |
|
| STABILITY |
Stable under ordinary conditions |
|
APPLICATIONS
|
|
4-(2-Aminoethyl)benzenesulfonamide is an
intermediate
to prepare sulfonylurea drugs like Glipizide (an antidiabetic
agent, chemically
1-cyclohexyl-3-[[p-(2-(5-methyl pyrazine carboxamido) ethyl]phenyl] sulfonyl]
urea). Sulfonylureas: a class of oral hypoglycaemic agents that exert hypoglycemic activity by stimulating the pancreas to secrete more insulin, active in type II diabetes only. Sulfonylureas have a phenyl ring nucleus with two branched chains in chemical structure. They can induce hypoglycemia which is potential life threatening side effect in case of over-dose. |
| SALES
SPECIFICATION |
|
APPEARANCE
|
white to off-white crystalline
powder |
|
PURITY |
98.0%
min
|
|
LOSS ON DRYING |
0.5%
max |
| TRANSPORTATION |
| PACKING |
|
| HAZARD CLASS |
|
| UN
NO. |
|
| OTHER INFORMATION |
|
Hazard Symbols: n/a, Risk Phrases: n/a, Safety Phrases: 24/25-28A-37-45 |
|
GENERAL DESCRIPTION OF AMIDE |
|
Amide is a group of organic chemicals with the general formula RCO-NH2 such as acetamide, where 'R' groups range from hydrogen to various linear and ring structures or a compound with a metal replacing hydrogen in ammonia such as sodium amide, NaNH2. Amide is formed from of ammonia (NH3) and a carboxylic acid in which a carbon atom is double bonded to oxygen and also to an hydroxyl group or by reaction of an acid chloride, acid anhydride, or ester with an amine. An amide is hydrolyzed to yield an amine and a carboxylic acid. The reverse of this process results in the loss of water and is used in nature to link amino acids to form proteins, the secondary structure of which is due in part to the hydrogen bonding abilities of amides. Sulfonamides are analogs of amides in which the atom double bonded to oxygen is sulfur rather than carbon. Polyamide is a polymer containing repeated amide groups, as in various kinds of nylon and polyacrylamides. |
