PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
AROMATIC AMINES / NITRO COMPOUNDS / DYES /
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
Stable under ordinary conditions
APPLICATIONS
- Michael addition
- Reduction
- Henry Reaction (Nitro-aldol reaction)
- Nef reaction
- O-Alkylation
- Cycloaddition
- Substitution, Elimination, Conversion reaction
- Alkylation, Acylation, and Halogenation
One of the most important aromatic amines is aniline, a primary aromatic amine replacing one hydrogen atom of a benzene molecule with an amino group. It is a pale brown liquid at room temperature; boiling at 184 C, melting at -6 C; slightly soluble in water and freely soluble in ether and alcohol. It causes serious industrial poisoning. The substance may have effects on the blood, resulting in formation of methaemoglobin. Repeated or prolonged exposures may be carcinogenic. Commercial aniline is obtained from nitrobenzene which is prepared from benzene with nitric acid by electrophilic substitution reaction or from chlorobenzene by heating with ammonia in the presence of copper catalyst. It is also obtained as a by-product of coal tar. In commerce the term of aniline oil blue refers to the pure one while aniline oil red indicates a mixture of aniline and toluidines with equimolecular weights.
Considerable quantity of aniline is converted into 4,4��-methylenedianiline (MDA) by the condensation reaction of formaldehyde with aniline in the presence of hydrochloric acid. MDA is is used as an epoxy curing agent, a corrosion inhibitor and molded plastics, and as an intermediate to prepare organic compounds used for polyurethane, spandex fibers, azo dyes, isocyanates and poly(amide-imide) resins. Other important aromatic amine compound as the starting material to produce polyurethane foam production is toluenediamine (TDA). TDA is the mixture of 2,4-diaminotoluene and 2,6-diaminotoluene, usually in a ratio of 80:20. Most of TDA is used in the manufacture of toluene diisocyanate (TDI), which is the predominant diisocyanate in the flexible foams and elastomers industries. TDI reacts with an alcohol to form urethane linkages. Other applications of TDA include to produce dyes, polyamides, antioxidants, hydraulic fluids, and fungicide stabilizers. Aniline is a starting moiety to prepare plant protecting agents. Examples include fenuron (CAS RN: 101-42-8), propham (CAS RN: 122-42-9), siduron (CAS RN: 1982-49-6), carboxin (CAS RN: 5234-68-4), fenfuram (CAS RN: 24691-80-3) and propachlor (CAS RN: 1918-16-7). Aniline is processed to produce a series of compounds being used in the rubber industry, e.g. diphenylguanidines, phenylenediamines mercaptobenzothiazoles, aniline ketones and etc. There are three isomers of phenylenediamine: ortho-, meta-, and para-phenylenediamine. They are low toxic diamines used as components of plastic composites and engineering polymers. They are used to produce aramid fibers, dyes including hair dyes, rubber chemicals (vulcanization accelerators and antioxidants), and pigments.
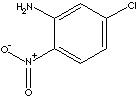
Aniline is the starting material in the dye manufacturing industry. It forms aniline colors when combined with other substances, particularly chlorine or chlorates. Aromatic amines are weaker bases reacting with strong acids to form amides. Anilide is an amide derived from aniline by substitution of an acyl group for the hydrogen of NH2. Acetanilide is thus obtained from acetic acid and aniline. Aniline is converted into sulfanilic acid which is the parent compound of the sulfa drugs. Aniline is also important in the manufacture of rubber-processing chemicals, explosives, plastics, antioxidants and varnishes. Amines take part in many kinds of chemical reactions and offer many industrial applications. Nitroaniline is used in the synthesis of dyes, agrochemicals, pharmaceuticals, rubber and plastic additives, photographic antifogging agents and coccidiostats. 5-chloro-2-nitroaniline is used as an intermediate for the syntheis of pharmaceuticals (especially for Fenbendazole - Anthelmintics) and other organic compounds.
APPEARANCE
CONTENT
99.0% min
MELTING POINT